More aggressive iguanas may need to be pinned down first. The use of a thick towel to control the tail and claws is useful. Gauntlets may be necessary for particularly aggressive large lizards or for those which may have a venomous bite. It is important to ensure that you do not use too much force when restraining the lizard, as those with skeletal problems, such as metabolic bone disease, may be seriously injured. In addition, lizards do not have a diaphragm and so overzealous restraint will lead to increasing pressure on the lungs.
Day geckos and other fragile species are best examined in a clear plastic container. Other geckos have easily damaged skin, so latex-free gloves and soft cloths should be used for examination. When handling small lizards, they may be cupped in the hand and their heads controlled by holding between the index finger and thumb to prevent biting.
It is important that lizards are never restrained by their tails. Many will shed their tails at this time, but not all of them will regrow. Green iguanas, e.g. will only regrow their tails as juveniles (less than 2.5–3 years of age). Once they are older than this, they will be left tailless. However, there are plenty of lizards that do not undergo autotomy, e.g. many agamids, chameleons, etc.
Vagovagal reflex
The vagovagal reflex can be used to place members of the lizard family into a trancelike state. The eyelids are closed and gentle digital pressure is applied to both eyeballs. This stimulates the autonomic parasympathetic nervous system resulting in a reduction in heart rate, blood pressure and respiration rate. Providing there are no loud noises or environmental stimulation, after 1–2 minutes the lizard may be placed on its side, front, back, etc. allowing radiography to be performed without using physical or chemical restraint. A loud noise or physical stimulation will immediately cause the lizard to revert to its normal wakeful state.
Snakes
Snakes are all characterised by their elongated form and absence of limbs. The danger areas for the handler are their teeth (and in the case of the most venomous species such as the viper family, their fang teeth), and, in the case of the constrictor and python family, their ability to asphyxiate their prey by winding themselves around the victim’s chest and neck.
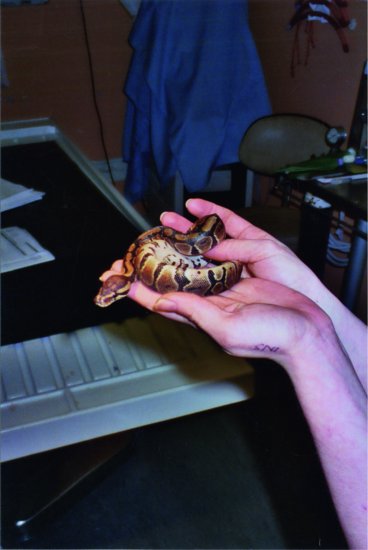
With this in mind, the following restraint techniques may be employed. Non-venomous snakes can be restrained by controlling the head initially. This is done by placing the thumb over the occiput and curling the fingers under the chin. Reptiles, like birds, have only one occipital condyle, so it is important to stabilise the occipito-atlantal joint. It is also important to support the rest of the snake’s body so that not all of the weight of the snake is suspended from the head. Allow the smaller species to coil around the handler’s arm, so the snake is supporting itself (Figure 19.2).
Figure 19.2 Allowing the snake to coil itself around the handler’s hand and arms is preferable to over-zealous restraint in non-aggressive species such as this small Royal python (Python regius).
In the larger species (longer than 10 feet) it is necessary to support the body length at regular intervals. This often requires several handlers. Indeed, it is vital to adopt a safe operating practice with the larger, constricting species of snake. A ‘buddy system’ should be operated wherein any snake longer than 5–6 feet in length should only be handled by two or more people. This ensures that if the snake was to enwrap one handler, the other could disentangle him or her by unwinding from the tail end first. Above all, it is important not to grip the snake too hard as this will cause bruising and the release of myoglobin from muscle cells. This can damage the glomerular filtration membrane in the kidneys.
Venomous snakes or very aggressive species may be restrained initially using snake hooks. These are 1.5–2 feet steel rods with a blunt shepherd’s hook on the end. They are used to loop under the body of a snake to move it at arm’s length into a container. The hook may also be used to trap the head flat against the floor before grasping it with the hand. Once the head is controlled safely the snake is rendered harmless. Exceptions include the spitting cobra family where handlers should wear plastic goggles, or a plastic face visor as they can spit poison into the prey or assailant’s eyes and mucous membranes causing blindness and paralysis.
Chelonia
The majority of Chelonia are harmless, although surprisingly strong. Mediterranean species, the tortoise may be held with both hands, one on either side of the main part of the shell behind the front legs. To keep the tortoise still for examination, it may be placed onto a cylinder or stack of tins, raising its legs clear of the table as it balances on the centre of the underside of the shell (plastron).
For aggressive species (e.g. snapping turtle and the alligator snapping turtle), it is essential that you hold the shell on both sides behind and above the rear legs to avoid being bitten. Chemical restraint is necessary in order to examine the head region in these species.
For the soft-shelled and aquatic species, soft cloths and latex-free gloves should be used to prevent damaging the shell.
Crocodylia
The Crocodylia include fresh and saltwater crocodiles, alligators, fish-eating gharials, and caimans. Their dangers to the handler lie in their impressively arrayed jaws and often their sheer size – an adult bull Nile crocodile may weigh many hundreds of kilograms.
Small specimens may be restrained by grasping the base of the tail in one hand whilst the other is placed behind the head. For slightly bigger specimens, a rope halter or noose may be tied around the snout so securing it closed. All of the major muscles in the crocodylian jaws are involved in closing not opening them, hence relatively fine rope or tape can be used to keep the mouth closed. The rest of the animal is restrained by pinning it to the ground.
Always approach crocodylians from head on, as their binocular vision is poor (although the alligator family does have some). Care should be taken when close to the crocodylian for head and tail movements both are directed at the assailant at the same time!
Much larger crocodiles require teams of people, with nets and snout snares in order to quickly clamp the jaws closed and to restrain the dangerous thrashing tail. Chemical immobilisation via dart guns is another option to be seriously considered.
Principles of chemical restraint
Chemical restraint is necessary for many procedures in reptile medicine, ranging from minor procedures such as extracting the head of a leopard tortoise or box turtle from its shell, to enabling a jugular blood sample to be taken or to carrying out coeliotomy procedures because of egg binding. Before any anaesthetic or sedative is administered, an assessment of the reptile patient’s health should be made. Considerations include
- Is sedation or anaesthesia necessary for the procedure required?
- Is the reptile suffering from respiratory disease or septicaemia?
- Is the reptile’s health likely to be made worse by sedation or anaesthesia?
Before discussing the administration of chemical restraint it is important to understand the reptilian respiratory system.
Overview of reptilian respiratory anatomy and physiology
The reptilian patient has a number of variations on the basic mammalian respiratory system.
The reptile patient has a glottis similar to the avian patient, which lies at the base of the tongue. This is more rostral in snakes and lizards and more caudal in Chelonia. At rest, the glottis is permanently closed, opening briefly during inspiration and expiration. In crocodiles, the glottis is obscured by the basihyal valve which is a fold of the epiglottis. This fold has to be deflected before they can be intubated (Bennett, 1998).
The trachea varies between orders. In Chelonia and Crocodylia, complete cartilaginous rings similar to those of the avian patient are found, with chelonians having a very short trachea. In some species, this trachea bifurcates into two bronchi in the neck. Snakes and lizards have incomplete C-rings, with snakes having a very long trachea.
The lungs of snake and lizard species are simple and elastic in nature. The left lung of most snakes is absent, or vestigial in the case of members of the boiid family. The right lung of snakes frequently ends in an air sac. Chelonian species have a more complicated lung structure, and the paired lungs sit dorsally inside the carapace of the shell. Crocodylian lungs are similar to mammalian lungs and are paired.
No reptile has a diaphragm, although crocodylians have a pseudodiaphragm which changes position with the movements of the liver and gut so pushing air in and out of the lungs. Most reptiles use intercostal muscles to move the ribcage in and out in a manner similar to birds. The exception to this is members of the order Chelonia. These species need to move their limbs, neck and head into and out of the shell in order to bring air into and out of the lungs.
Some species can survive in oxygen-deprived atmospheres for prolonged periods – chelonian species may survive for 24 hours or more, and even green iguanas may survive for 4–5 hours. This makes induction of anaesthesia via inhalation of a gaseous anaesthetic agent almost impossible in these animals. The stimulus for respiration in reptiles is therefore predominantly driven by lowered pO2 rather than elevated pCO2 and many reptiles when breath-holding show right-to-left shunting of blood in the heart so by-passing the lungs.
Many snakes have both intrapulmonary chemoreceptors and stretch receptors whereas many chelonians have only stretch receptors, hence when there are high levels of carbon dioxide, snakes can override the volume related feedback and continue to breathe. Hypercapnia tends to increase the tidal volume by suppressing the stretch receptors. Hypoxia increases breathing frequency by reducing or eliminating the non-breathing periods and these effects are increased at higher environmental temperatures.
In snakes, the central control of respiration can also override the stretch receptors allowing them to control breathing even when constricting prey or swallowing large prey which may impinge on the lungs.
Pre-anaesthetic preparation
Weight measurement
This is important for accuracy as some species of reptile may be very small. Scales accurate to 1 g are therefore advised for smaller reptiles to ensure correct dosage.
Blood testing
It may be advisable to test biochemical and haematological parameters before administering chemical immobilising drugs. Blood samples can be taken from
- Jugular vein in Chelonia
- Dorsal tail vein in Chelonia
- Ventral tail vein in snakes, Crocodylia and lizards
- Palatine vein or cardiac puncture in snakes (although they frequently need to be sedated or anaesthetised to collect blood from these routes).
Fasting
Fasting is necessary in snakes to prevent regurgitation and pressure on the lungs or heart. It is advisable to ensure that no prey has been offered in the two days prior to anaesthesia. Other reptiles require less fasting, e.g. chelonians rarely if ever regurgitate. However, it is important not to feed live prey to insectivores such as leopard geckos 24 hours prior to an anaesthetic as the prey may still be alive when the reptile is anaesthetised.
Pre-anaesthetic medications
Premedications are used to provide cardiopulmonary and central nervous system stabilisation, a smooth anaesthetic induction, muscle relaxation, analgesia and a degree of sedation.
Antimuscarinic medications
Atropine (0.01–0.04 mg/kg IM) or glycopyrrolate (0.01 mg/kg IM) may be used to reduce oral secretion and to reduce bradycardia; however, these are not usually of concern in reptiles. Indeed, antimuscarinics may increase the thickness of mucus secretions, leading to more rapid blocking of the airways. In addition, in herbivores it can result in prolonged periods of ileus.
Tranquilisers
Acepromazine (0.1–0.5 mg/kg IM) may be given one hour before anaesthetic induction to reduce the levels of anaesthetic required, as can diazepam (0.22–0.62 mg/kg IM in alligators) and midazolam (2 mg/kg IM in turtles) (Bennett, 1998).
Alpha-2 adrenoceptor stimulants
Xylazine at 1 mg/kg can be used 30 minutes prior to ketamine in crocodylians to reduce the dose of ketamine needed. Medetomidine, used at doses of 100–150 mg/kg, markedly reduces the dose of ketamine required in chelonians, and has the advantage of being reversible with atipamezole at 500–750 mg/kg. They both create a drop in blood pressure and cardiac output and so should be used with caution in debilitated reptiles.
Opioids
Butorphanol (at 0.4 mg/kg IM) can be administered 20 minutes before anaesthesia; this provides analgesia and reduces the amount of anaesthetic required. This drug may be combined with midazolam at 2 mg/kg. It does not have any sedation or general anaesthetic properties but does seem to have anaesthetic sparing properties allowing reduced levels of gaseous anaesthetic to be used.
Fluids
Fluid therapy is very important, and correction of fluid deficits should be attempted prior to surgery. Maintenance levels in reptile patients have been quoted as 25–30 mL/kg/day (see chapter 22 for more information on fluid therapy in reptiles).
Induction of anaesthesia
It should be noted that reptiles should never be immobilised by chilling or cooling them down. This does not provide analgesia and has serious welfare implications.
Injectable agents
Table 19.1 describes the advantages and disadvantages of injectable anaesthetic agents.
Table 19.1 Advantages and disadvantages of injectable anaesthetics.
| Advantages | Disadvantages |
| Ease of administration | Recovery often dependent on organ metabolism |
| Prevention of breath-holding on induction | Difficult to reverse rapidly |
| Reduced costs | Often prolonged recovery times |
| Easy to administer | Muscle necrosis at site of injection |
| Low risk to anaesthetist |
Dissociative anaesthetics
Ketamine
Recommended levels range from 22 to 44 mg/kg IM for sedation to 55–88 mg/kg IM for surgical anaesthesia. Lower levels are needed if combined with a premedicant such as midazolam or medetomidine (Bennett, 1996). Doses in excess of 110 mg/kg will produce profound bradycardia and the death of the reptile.
Effects are seen in 10–30 minutes but may take anything up to 4 days to wear off, particularly at low environmental temperatures. Its main use is therefore at the lower dose range, to allow sedation, facilitate intubation and maintenance of gaseous anaesthesia in species such as chelonians that hold their breath during gaseous induction. Doses of 5–10 mg/kg have been used in Chelonia to allow extraction of the head from the shell.
It is, however, frequently painful on administration. Also, because ketamine is excreted by the kidneys, it is recommended that it is administered in the cranial half of the body. This is because blood from the caudal half of the body travels to the kidneys before returning to the heart and the anaesthetic may thus be excreted before it has a chance to work. In addition, as it is actively excreted by the kidneys, its use in reptiles with renal disease will result in prolonged recovery periods.
Ketamine (5 mg/kg) may be combined with medetomidine (100 ug/kg) to facilitate intubation of small reptiles (<2 kg) or at 7.5 mg/kg ketamine plus 75 ug/kg medetomidine for reptiles >2 kg.
Other injectable anaesthetics
Alfaxalone
This can be used to aid induction, allowing intubation within 3–5 minutes when administered intravenously. It may be administered intramuscularly but induction takes longer via this route (25–40 minutes). Doses of 9 mg/kg have been advised and it appears in many reptiles not to be capable of inducing full anaesthesia but rather permitting intubation and causing immobilisation (Sheelings et al., 2010).
Propofol
Propofol produces rapid induction and recovery. Its advantages include a short elimination half-life and minimal organ metabolism, making it relatively safe to use in debilitated reptiles which often have some liver damage.
Its disadvantage is that it requires intravenous access, although use of the intraosseous route is useful in green iguanas at a dose of 10 mg/kg. Propofol also produces a transient period of apnoea and some cardiac depression. In this situation, intubation and positive pressure ventilation is necessary.
Doses of 10–15 mg/kg in chelonians given via the dorsal coccygeal (tail) vein have successfully induced anaesthesia in under 1 minute. This allows intubation and maintenance on a gaseous anaesthetic if required. Alternatively, propofol can be used alone providing a period of anaesthesia of 20–30 minutes. For giant species of Chelonia, lower doses of 1–2 mg/kg IV/IO have been used.
Depolarising muscle relaxants
Succinylcholine
This is a neuromuscular blocking agent and produces immobilisation without providing analgesia. Therefore, it should only be used to aid the administration of another form of anaesthetic or for transportation, and not as a sole source of anaesthesia. Recovery is dependent on liver metabolism and its use in animals with possible liver disease should be avoided.
It can be used in giant Chelonia at doses of 0.5–1 mg/kg IM and will allow intubation and conversion to gaseous anaesthesia. Crocodilians can be immobilised with 3–5 mg/kg IM, with immobilisation occurring within 4 minutes and recovery in 7–9 hours. Respiration usually continues without assistance at these doses, but is important to have assisted ventilation facilities to hand as paralysis of the muscles of respiration can easily occur.
Reversal of succinylcholine is not possible and the patient must be ventilated until the drug has been excreted.
Gallamine
This has been used in crocodiles (0.3–1.5 mg/kg) to achieve immobility in 15–30 minutes with a recovery time of 1.5–3 hours. Its advantage over succinylcholine is that it is reversible with neostigmine (0.25 mg/kg).
Gaseous agents
The gaseous anaesthetics used for induction will be discussed in the next section on maintenance of anaesthesia, however, a table listing their advantages and disadvantages is presented in Table 19.2.
Table 19.2 Advantages and disadvantages of gaseous anaesthetic.
| Advantages | Disadvantages |
| Ease of administration via face mask | Breath-holding (chelonia particularly) |
| Pain free | Environmental pollution |
| Minimal tissue trauma | Health risk to anaesthetist Risk with dangerous reptiles during handling |
Maintenance of anaesthesia
Injectable agents
Dissociative anaesthetics
Ketamine
Ketamine may be used on its own for anaesthesia at doses of 55–88 mg/kg IM. It is worthwhile noting though that as the dosages get higher the recovery time also increases, and in some cases it can be as long as several days. Also, doses above 110 mg/kg will cause respiratory arrest and bradycardia.
Ketamine may be combined with other injectable agents to provide surgical anaesthesia. Examples of these combinations include
- Midazolam at 2 mg/kg IM with 40 mg/kg ketamine in turtles (Bennett, 1996)
- Xylazine at 1 mg/kg IM, given 30 minutes prior to 20 mg/kg ketamine in large crocodiles (Lawton, 1992)
- Medetomidine at 100 mg/kg IM with 50 mg/kg ketamine in kingsnakes (Malley, 1997).
Other injectable anaesthetics
Propofol
Propofol may be used to give 20–30 minutes of anaesthesia after administration, allowing minor procedures such as wound repair, intraosseous or intravenous catheter placement, or oesophagostomy tube placement to be carried out.
It may be topped up at 1 mg/kg/min IV/IO, but apnoea is extremely common and intubation and ventilation with 100% oxygen is required.
Alphaxalone
This can be used for induction and also for short periods of anaesthesia (average 25 minutes) at 9 mg/kg IV/IO. Topping up of the anaesthetic allows maintenance of light anaesthesia for longer procedures but caution should be observed as it may not produce enough anaesthesia to allow invasive procedures in all species. Kischinovsky and Bertelsen (2011) showed that 30 mg/kg was required in green iguanas to achieve surgical anaesthesia, but many became apnoeic. Recovery times are often 1–4 hours.
Gaseous agents
Isoflurane
Isoflurane is the gaseous maintenance anaesthetic of choice (see also Table 19.2). Isoflurane is minimally metabolised in the body (0.3%) and has a very low blood–gas partition coefficient (1.4 compared with 2.3 for halothane in human trials). This means that it has a very low solubility in blood, so as soon as administration is stopped the reptile starts to recover, excreting it from the lungs. In addition, it has low fat solubility and so is not stored. Isoflurane still has excellent muscle relaxing properties and is a good analgesic during anaesthesia. Apnoea precedes cardiac arrest, unlike the case with halothane anaesthesia.
It can be used to induce anaesthesia in those species not exhibiting breath- holding at levels of 4–5%, by induction chamber. It is also possible to adapt the cases of 20 mL and 60 mL syringes to form long, thin face masks to induce snakes. Isoflurane can then be used to maintain anaesthesia, preferably via endotracheal tube, at levels from 2–3% depending on the procedure.
Sevoflurane
As with isoflurane, this drug may be safely used. It is highly insoluble in the bloodstream though, and so ventilation rates may need to be increased above the usual 4–6 breaths per minute to maintain anaesthesia. In this author’s experience, it cannot be used to maintain anaesthesia alone in certain species of reptiles (leopard geckos, bearded dragons and green tree pythons to name a few) and so care should be taken in selecting this gas particularly when using a different induction agent as once the induction agent wears off the patient may wake up!
Induction and maintenance levels of sevoflurane are higher than for isoflurane being typically 6–8% and 3–4% respectively.
Nitrous oxide
Nitrous oxide can be used in conjunction with isoflurane, reducing the percentage of gaseous anaesthetic required for induction and maintenance of anaesthesia. Its other advantages include good muscle relaxation and excellent analgesic properties, making it useful in orthopaedic procedures.
Disadvantages of nitrous include its tendency to accumulate in hollow organs. This may prove a problem for herbivorous reptiles as they often have capacious hind guts and nitrous oxide can accumulate there. Nitrous oxide also requires some organ metabolism for full excretion and so may be a problem in a seriously diseased patient. It also prolongs anaesthetic recovery times by up to 50%.
Aspects of gaseous anaesthesia maintenance for reptiles
Inhalant gaseous anaesthesia is becoming the main method of anaesthetising reptiles for prolonged procedures. The reptile patient should preferably be intubated to allow the inhalant anaesthetic to be delivered in a controlled manner.
Intubation
Intubation is straightforward in reptiles as they do not have an epiglottis and the glottis, which acts as the entrance to the trachea, is relatively cranial in the majority of species. It is useful to note that the glottis is kept closed at rest, so the operator must wait for inspiration to occur to allow intubation. Reptiles produce little or no saliva when at rest or not eating, so blockage of the tube is uncommon.
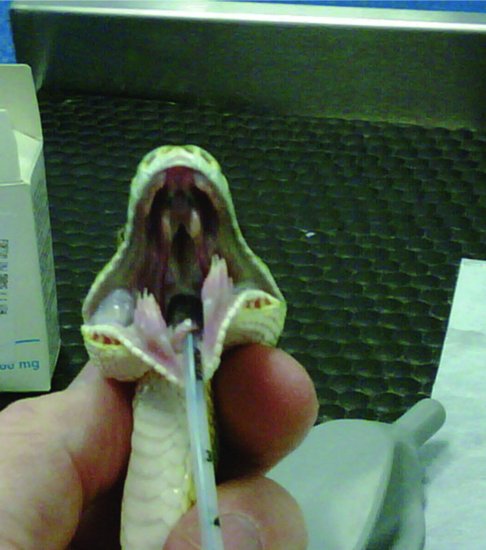
In snakes, the glottis sits rostrally on the floor of the mouth just caudal to the tongue sheath and is easily visible when the mouth is opened (Figure 19.3). Intubation may be performed in the conscious patient if necessary, as reptiles do not have a cough reflex. The mouth is opened with a wooden or plastic tongue depressor and the endotracheal tube inserted during inspiration. Alternatively, an induction agent may be given and then intubation attempted.
Figure 19.3 Intraoral views after intubation of a green tree python (Morelia viridis) showing glottal tube.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


