Chapter 4 Equine Ocular Adnexal and Nasolacrimal Disease
Clinical Anatomy and Physiology
Eyelids
Equine eyelids conform to the same basic anatomy of other domestic animals. The three basic layers of the superior and inferior eyelids from external to internal are (1) the skin with its haired and associated sebaceous glands; (2) a musculofibrous layer consisting of muscle, connective tissue, and tarsal plate; and (3) the palpebral conjunctiva.1 The eyelids are critical to ocular health in a number of ways. The blink reflex in response to tactile, chemical, or thermal stimuli of the skin, vibrissae, cilia, conjunctiva, or cornea serves to protect the eye. Reflex blinking (and tearing) also helps remove any foreign particulate matter from the eye. The eyelids produce portions of the preocular tear film (e.g., meibomian glands produce lipid, conjunctival goblet cells produce mucin) and facilitate tear distribution across, and clearance from, the corneal surface. Finally, the eyelids regulate the amount of light that enters the eye.
On the skin surface, the equine eye has three types of hair: vibrissae, cilia, and dermal hair (Fig. 4-1). Typically, two to four vibrissae are present 2 to 3 cm dorsal to the medal canthus and 8 to 10 vibrissae emerge approximately 1 cm ventral to the inferior eyelid and run parallel to the interpalpebral fissure. The function of these relatively stiff hairs is to provide tactile stimuli via cranial nerve (CN) V; touching the vibrissae will usually prompt eyelid closure and therefore should be avoided during the ocular examination. Eyelid cilia originate just external to the opening of the meibomian glands on the palpebral margin and may be associated with other modified skin glands (e.g., glands similar to those of Zeis and Moll). Equine eyelid cilia are large and numerous on the superior eyelid but few or absent on the inferior eyelid (see Fig. 4-1).2 Eyelid cilia primarily function to protect the cornea, shield the eye from light, and provide tactile sensory input. Fine, soft dermal hairs cover the eyelid surface in variable degrees of density. The area within 0.5 mm of the eyelid margin generally has few dermal hairs.
The tarsus or tarsal plate is a narrow, dense layer of connective tissue located between the palpebral conjunctiva and the eyelid skin surface. Both superior and inferior eyelids possess a tarsal plate, but it is more fully developed in the superior eyelid. The tarsi provide structure and rigidity to the eyelids.3 Horses possess a well-defined orbitopalpebral sulcus, most evident in the superior eyelid (Fig. 4-2).1 This sulcus forms a fold of skin that delineates the eyelid into an orbital portion and a tarsal portion. The orbital portion lies between the dorsal margin of the bony orbit and the globe, and the tarsal portion overlies the globe itself. Traumatic superior eyelid lacerations are often observed to tear along this sulcus and necessitate meticulous surgical repair.
The tarsal, or meibomian, glands are a row of well-developed sebaceous eyelid glands (Fig. 4-3). Meibomian glands are arranged in a single row running parallel to the lid margins and are embedded within the tarsal plate. The glands open directly onto the eyelid margins in a row of evenly spaced orifices collectively known as the gray line, an important surgical landmark. The meibomian glands secrete the lipid layer of the precorneal tear film. Other glandular structures in the equine eyelid (see Fig. 4-3) include sebaceous glands associated with cilia (i.e., Zeis-like glands) and modified sweat glands (i.e., Moll-like or ciliary glands).3–5
The eyelids are well vascularized via longitudinal vessels that run parallel to the eyelid margins and originate from the malar, superficial temporal, and ventral palpebral arteries.3,4 The excellent blood supply provides good healing of the eyelids after surgery or traumatic laceration. The eyelids have substantial lymphatic drainage, primarily to ipsilateral parotid and mandibular lymph nodes. This anatomic feature is of clinical significance in periocular neoplasia, especially squamous cell carcinoma, because the primary site of metastasis is to these draining lymph nodes.6
Eyelid musculature, innervation, function, and clinical descriptions of periocular motor and sensory nerve blocks have been reviewed in Chapter 1. Briefly, motor innervation to the eyelids is from CN III (the oculomotor nerve) and CN VII (the facial nerve). Sensory innervation to all aspects of the equine eyelid is from CN V (the ophthalmic branch of the trigeminal nerve) (Fig. 4-4). The muscles of the eyelids consist of the levator anguli oculi medialis, levator palpebrae superioris, frontalis, orbicularis oculi, retractor anguli oculi, malaris, and Müller’s muscle (Fig. 4-5).1 The orbicularis oculi muscle, innervated by the palpebral branch of CN VII, surrounds the palpebral fissure to form a sphincter that closes the eyelids. The levator palpebrae superioris, innervated by CN III, originates in the posterior orbit, inserts into fibers of the orbicularis oculi muscle of the upper eyelid, and acts to elevate the upper eyelid. The malaris muscle, innervated by a branch of CN VII, attaches to the orbicularis oculi fibers of the lower eyelid and functions to lower the inferior eyelid. Müller’s muscle is smooth muscle innervated by the sympathetic nervous system. It is oriented perpendicular to the eyelid margin and provides the “tone” to the tarsus. Loss of sympathetic tone to Müller’s muscle occurs in Horner’s syndrome and results in upper-eyelid ptosis.7–9 Other muscles that control equine eyelid movement include the retractor anguli muscle that retracts and anchors the lateral canthus and the levator anguli oculi medialis and frontalis muscles that provide slight elevation of the upper eyelid (Table 4-1).
Table 4-1 | Equine Eyelid Muscles: Innervation and Function
| MUSCLE | INNERVATION | FUNCTION |
|---|---|---|
| Orbicularis oculi | CN VII (Facial) | Eyelid closure |
| Levator palpebrae superioris | CN III (Oculomotor) | Elevate the superior eyelid |
| Malaris | CN VII (Facial) | Depress the inferior eyelid |
| Müller’s | Sympathetic | Elevate the superior eyelid |
| Retractor anguli oculi | CN VII (Facial) | Lengthen palpebral fissure |
| Levator anguli oculi medialis | CN VII (Facial) | Lengthen and elevate medial canthus |
| Frontalis | CN VII (Facial) | Elevate the superior eyelid |
CN, Cranial nerve.
In the adult healthy horse, the palpebral fissure is open approximately 36 to 51 mm (mean of 43.5 mm) in the horizontal plane (temporal to nasal) and approximately 25 mm at its widest vertical dimension.10 The lateral canthus denotes the region where the superior and inferior eyelids adjoin temporally, and the medial canthus is where the superior and inferior eyelids meet nasally (see Fig. 4-1). Structure of the equine eyelids is maintained not only by the palpebral muscles but also by palpebral ligaments. The medial canthal (palpebral) ligament is a firm, ligamentous band anchoring the medial canthus to the dense periorbital fascia and the periosteum of the medial orbital rim (see Fig. 4-5). The lacrimal sac lies just deep and ventral to this ligament. The anguli oculi vein runs just medial to the lacrimal sac. The lateral canthal (palpebral) ligament is formed by the junction of the temporal extremities of the superior and inferior eyelid tarsal plates and serves to anchor the lateral canthus to the lateral margin of the orbit. Normal eyelid position is necessary for adequate drainage of the tear film. Closure of the lids occurs from lateral (temporal) to medial (nasal). Normal eyelid closure distributes the preocular tear film over the corneal surface and mechanically facilitates tear drainage out the nasolacrimal outflow tract.
The lacrimal caruncle lies deep to the medial canthus and external to the third eyelid (see Fig. 4-1). This structure varies somewhat in size among horses and may contain modified sebaceous and sweat glands. If enlarged, the lacrimal caruncle may protrude and prevent the eyelids from conforming as well to the medial aspect of the cornea compared to the lateral aspect.2 Conjunctiva, a nonkeratinized squamous epithelium, lines the inner aspect of the superior and inferior eyelids (i.e., palpebral conjunctiva) (see Fig. 4-3).
The third eyelid, or nictitating membrane (see Fig. 4-1), originates in the ventromedial aspect of the orbit and moves passively in the dorsolateral direction with retropulsion of the globe or as the globe is retracted during blinking. The third eyelid is covered with conjunctiva that is richly endowed with goblet cells. A T-shaped cartilage lies within the body of the third eyelid and conforms to the corneal curvature of the globe. The cartilage provides structural support along the leading margin of the third eyelid via a horizontal “arm” and through the center of the third eyelid by its vertical component (Fig. 4-6). The base of the third eyelid contains a lacrimal gland (tubuloacinar) that produces a portion of the aqueous component of the tear film (see Fig. 4-6).11 A large fat pad lies ventral to the gland of the third eyelid.4
Conjunctiva
Conjunctiva is composed of nonkeratinized stratified columnar cells that are continuous with the corneal epithelium. The conjunctiva consists of three primary anatomic regions that are contiguous with each other: the palpebral conjunctiva, the bulbar conjunctiva, and the fornix. The palpebral conjunctiva lines the superior and inferior eyelids (see Fig. 4-3). The bulbar conjunctiva covers the anterior aspect of the globe to the limbus. The conjunctival fornix (superiorly) and cul-de-sac (inferiorly) are the junctions between those areas where the superior and inferior palpebral and bulbar conjunctiva meet, respectively.4,12 In the adult horse, the fornix (dorsally) is located approximately 3 cm from the limbus, while the cul-de-sac (ventrally) is approximately 2.5 cm from the limbus.4 Conjunctiva also covers the anterior and posterior surfaces of the third eyelid. Conjunctiva is richly vascularized, variably pigmented (especially the bulbar conjunctiva), and has many goblet cells that contribute to the preocular tear film.13 In horses, it is common to visualize aggregates of lymphoid follicles located perilimbally (Fig. 4-7). Likely, these lymphoid follicles represent part of the equine conjunctiva-associated lymphatic tissue (CALT) that has been shown to be integral to the ocular immune response in other species.14–17
Nasolacrimal System
The nasolacrimal system of the horse comprises both secretory and drainage portions. The lacrimal gland, a tubuloacinar gland, is located dorsolateral to the globe and has 3 to 5 ductules that transport lacrimal fluid from the gland to the dorsal conjunctival fornix.3 The gland of the third eyelid surrounds the base of the cartilage of the nictitans. The percentage of aqueous tears produced by the gland of the third eyelid in horses is not known; however, one study found that horses with surgically resected third eyelids did not have differences in basal (tested via Schirmer tear test II) or reflex (tested via Schirmer tear test I) tear production compared to normal horses.18 In general, tear production in horses is robust, and copious reflex tearing can occur in response to ocular irritation or pain. Very few cases of keratoconjunctivitis sicca in horses have been reported in the veterinary literature.19–22
The nasolacrimal drainage apparatus of the horse may become obstructed due to a foreign body, calcification, dentition abnormalities, inflammation/infection, or neoplastic processes.2,23,24 The eyelid lacrimal puncta are oval openings, approximately 2 mm in diameter and located 8 to 9 mm lateral to the medial canthus of the superior and inferior eyelids.25 Through these puncta, lacrimal fluid enters the canaliculi, 3- to 4-mm diameter tubes that join to form the main nasolacrimal duct (Fig. 4-8). The equine nasolacrimal “sac” is much less developed than in human beings and anatomically represents only a slight dilation of the outflow tract where the two upper and lower canaliculi join.25 The nasolacrimal duct continues distally 7 to 8 cm within the osseous lacrimal canal of the lacrimal and maxillary bones (Fig. 4-9).25 The duct narrows slightly immediately prior to its exit from the lacrimal canal of the maxilla bone (see Figs. 4-8 and 4-9). Externally, the course of nasolacrimal duct through the maxillary bone follows a line from the medial canthus to the infratrochlear foramen.25 The duct then courses in the submucosa along the nasal wall of the lateral aspect of the middle meatus, then dips ventrally as it courses in the ventral nasal fold. The duct curves laterally over the basal process of the incisive bone to open at a 3- to 4-mm, oval nasolacrimal orifice on the ventral floor of the nasal vestibule (Fig. 4-10).25 Two or more nasolacrimal openings are very common in the nasal vestibule of horses. The total length of the nasolacrimal duct is approximately 24 to 30 cm long in the average adult horse.4
Economic Impact of Ocular Adnexal Disease on the Equine Industry
Eyelid and nasolacrimal diseases are among the most common ocular diseases in horses. According to a U.S. Department of Agriculture 1998 study, the overall prevalence rate for eye diseases was 7.4% in horses older than 6 months of age.26 Although this study did not differentiate among ocular problems, eyelid and nasolacrimal diseases likely constitute a large majority of these cases. For example, eyelid disease is the second most common presenting complaint of horses presented to the Veterinary Medical Teaching Hospital at the University of Missouri. It is reasonable to conclude that equine adnexal disease has a substantial economic impact on the equine industry in general.
Congenital Ocular Adnexal Disease
Entropion
Entropion, or turning in of the eyelid margins, is the most common ocular abnormality in foals (Fig. 4-11).27,28 Most commonly, the inferior temporal eyelid is affected, but both eyelids may be entropic. The most common cause of entropion in foals is secondary to loss of orbital fat (cachexia) or dehydration due to systemic illness or maladjustment. The loss of orbital contents results in globe retraction, thus allowing the eyelids to roll inward. The in-turned eyelids will result in corneal irritation and possibly ulceration from mechanical abrasion by the cilia and/or skin hair. Ocular discomfort from the anatomic component of entropion results in spastic entropion, where the globe retracts secondary to pain, further exacerbating the degree of entropion already present.

Figure 4-11 Entropion secondary to enophthalmos in a foal with neonatal maladjustment syndrome.
(Photograph courtesy Dr. Riccardo Stoppini.)
Differential Diagnosis
The most common cause of entropion in neonatal foals is systemic disease or maladjustment, as previously stated. However, some horses may be genetically predisposed, such as Thoroughbreds29 and Quarter Horses.2 The mode of inheritance or prevalence of this eyelid defect is not known. Other causes of entropion include microphthalmia, eyelid trauma, scarring (cicatricial entropion), or any disease causing prolonged blepharospasm.2,30,31 Entropion in foals must be differentiated from other congenital eyelid abnormalities such as ankyloblepharon, coloboma, dermoids, and cilia abnormalities (Table 4-2).
Treatment
Medical therapy for entropion consists most commonly of corneal protection using frequent (every 4 to 6 hours) application of topical ophthalmic ointment on the affected eyes and appropriate therapy for any underlying systemic illness. Benign ophthalmic lubricating ointment (applied in conjunction with temporary tacking sutures [see next section]) is often sufficient, providing no corneal ulceration is present. If ulcerative keratitis is present, appropriate management for corneal ulcer and secondary uveitis should be instituted. Systemic nonsteroidal medications and topical atropine should be used with caution in these sick foals. Ophthalmic preparations containing steroids should be avoided. The recumbent nature of sick foals will predispose them to corneal ulceration even if fluorescein stain is negative at initial presentation to the veterinarian. Various materials have been reported for use to correct entropion. Substances including saline, penicillin G, silicone, hyaluronic acid, and liquid paraffin have been injected into eyelid margins to cause temporary eversion.4 Although these materials may be effective, the need for repeated injections, eyelid inflammation, and the potential for scarring make this technique undesirable.
Temporary surgical repair of the entropion is almost always needed to correct both the anatomic and spastic components (i.e., entropion→ corneal irritation→ blepharospasm and globe retraction→ further exacerbation of entropion→ etc.). Temporary surgical repair allows the cornea to heal without mechanical irritation and does not carry with it the risk of permanent eyelid disfigurement from overcorrection. Temporary entropion repair is typically achieved with the foal sedated and placement of everting (tacking) sutures in a vertical mattress simple interrupted pattern. Nonabsorbable 4-0 to 5-0 monofilament suture is recommended. Silk has excellent knot security and can be used but may result in mild to moderate eyelid tissue reaction. When placing temporary tacking sutures, the “law of bisection” is recommended such that the first suture is passed in the center of the entropic eyelid, beginning 1 to 2 mm away from the eyelid margin perpendicular to the eyelid. The clinician should take care to avoid passing the suture through the eyelid margin, as sutures may be torn out, resulting in a large eyelid defect. Next, an additional bite of skin ventral to the original bite is taken. The distance between the two suture bites will determine the amount of eyelid eversion achieved once the vertical mattress suture is securely tied. If, for example, a large eversion/imbrication is necessary to correct the entropic eyelid, a 1- to 2-cm distance between the dorsal and ventral bites of the vertical mattress suture may be required. Additional sutures are placed in an identical fashion adjacent (usually 5 mm between sutures) to the central suture (Fig. 4-12). The number of sutures required will depend on the length of entropic eyelid. Knots should be secured with four throws, and the suture end nearest to the cornea should be cut short while the opposite end left longer to facilitate suture removal. Applying a drop of cyanoacrylic adhesive to the knots will help ensure that they will stay in place on the eyelid skin. After temporary tacking has been performed, elicit a palpebral reflex to ensure that the foal can blink normally. If the foal is lagophthalmic (e.g., unable to close the eyelids completely) after temporary tacking, removal of one or more sutures may be necessary to prevent exposure keratitis. Sutures typically remain in place for 2 to 4 weeks in most foals.
Permanent Surgical Repair for Entropion
The most common surgical procedure for entropion involves an elliptical skin excision known as a Hotz-Celsus procedure (Fig. 4-13). This surgical procedure permanently everts the eyelid margin by removing a segment of skin equal to the amount of skin that is entropic. Some practical tips to help ensure success with a simple Hotz-Celsus include the following:
Other Congenital Diseases of the Adnexa
Congenital adnexal diseases including ankyloblepharon, eyelid agenesis (Fig. 4-16), coloboma, dermoids (Fig. 4-17), and cilia abnormalities have been reported.4,27,28,30,32–34 Features of these conditions are listed in Table 4-2. Congenital ankyloblepharon, or fusion of the eyelids together, after birth is not normal in foals, since horses have fully developed adnexa at birth.4 Separation of the eyelids should be attempted first manually, then using blunt dissection starting at the medial canthus.32 Occasionally, foals will present blind with significant congenital ophthalmic disease that affects both the adnexa and the globe (Fig. 4-18).35
Congenital Diseases of the Nasolacrimal System
Epiphora
Epiphora, or overflow of tears, can occur from excessive lacrimation due to ocular irritation, an eyelid abnormality (e.g., entropion), or obstruction of the nasolacrimal duct. Obstructions of the nasolacrimal duct occur as a result of, or develop secondary to, bacterial infections (i.e., dacryocystitis) and are associated with profound mucopurulent discharge (Fig. 4-19). Congenital deficiency in tear production or keratoconjunctivitis sicca (KCS) has not been reported in horses but may occur. In fact, spontaneous development of KCS in horses, in general, is very uncommon, and the KCS that has been reported is typically secondary to trauma or other inflammatory disorders or endocrinopathies.19–22
Nasolacrimal Duct Atresia
The most common congenital anomaly of the nasolacrimal duct is nasolacrimal duct atresia.4,27,30,32–34 The most common defect is an imperforate nasal punctum, although eyelid punctal atresia or incomplete formation of the duct can occur anywhere along its course (see Figs. 4-8 and 4-9). Mild to moderate unilateral or bilateral epiphora is initially observed, but by 4 to 6 months of age, the predominant clinical sign is severe mucopurulent ocular discharge (see Fig. 4-19) due to the development of secondary bacterial dacryocystitis. Diagnosis is made by direct observation of the nasal vestibule (i.e., lack of punctal openings) and inability to irrigate the nasolacrimal system from the eyelid puncta. Also, swelling of the nasal epithelium distally, near the area of normal punctal opening, may also suggest atresia.4,36,37 Rarely, eyelid punctal atresia may occur, resulting in failure of nasolacrimal irrigation (retrograde) and conjunctival swelling.30
Differential Diagnosis
It is important to differentiate acquired obstructive diseases of the nasolacrimal duct from true atresia. Acquired obstructions generally have open, visible punctal openings (see Fig. 4-10) but an inability to irrigate the nasolacrimal system. Because nearly all cases of nasolacrimal punctal atresia (either eyelid or nasal) have secondary dacryocystitis, bacterial culture and sensitivity should be submitted to appropriately manage concurrent infection. Contrast radiography (e.g., dacryocystorhinography [DCR]; see Chapter 1 for description of the technique) can be very helpful in determining the exact location of the obstruction (Fig. 4-20). A diagnostic DCR may help determine the type of surgery that may be needed (i.e., incision of duct and stent placement versus dacryocystorhinotomy/sinostomy) and the long-term prognosis.38
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






















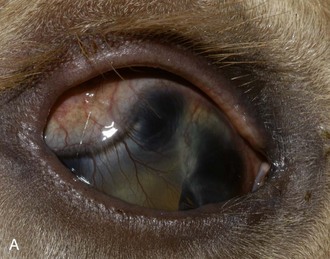


 -year-old Quarter Horse with a 20-month history of copious mucopurulent discharge from the left eye. Diagnostic testing confirmed imperforate left nasolacrimal duct punctum.
-year-old Quarter Horse with a 20-month history of copious mucopurulent discharge from the left eye. Diagnostic testing confirmed imperforate left nasolacrimal duct punctum.