• Ataxia at birth makes this distinguishable from other conditions such as cerebellar abiotrophy that develop later. Definitive diagnosis is only possible at post-mortem. The cerebellum is grossly and histopathologically abnormal. • No treatment is possible but less severely affected animals may survive with a satisfactory quality of life. • As it has not been shown to be an inherited condition continuing to breed from the parents is unlikely to result in the birth of another similarly affected foal. • The condition may be suspected on the basis of clinical signs and suitable breed but currently can only be confirmed at post-mortem examination. A cerebellum to whole brain weight ratio of less than 10% confirms relative smallness of the cerebellum. In some cases transverse cerebellar sections show an obvious loss of white matter. • Newer imaging modalities, where available, may be useful for ante-mortem diagnosis but to date there are no reports of their use. • Histopathology shows degeneration of Purkinje cells, atrophy of cerebellar folia and loss of the external granular layer. • Differential diagnosis: Cerebellar abiotrophy (must be differentiated from other rare congenital tremor syndromes such as in utero exposure to organophosphates or Arnold-Chiari malformation although these may also have other neurological signs); acquired diseases (abscessation and migrating parasites). • There are no treatment options and clinical signs can be expected to progress. • The disease can be confirmed by plain radiography. Malformations of the occiput, C1 and C2 are present including atlanto-occipital fusion and hypoplasia of the dens. Healed atlanto-axial fractures can mimic OAAM. • No treatment is indicated in most cases and since the disease is most likely inherited in the Arabian, the owners should be counseled not to breed close family lines. • Laminectomy has been used to alleviate spinal cord compression and clinical signs. • Single or multiple epileptiform seizures. Frequently, these seizures are not seen and all that is noticed is evidence of self-trauma. If seen these seizures usually follow a consistent pattern which is individual for each foal. • Prodromal signs and postictic signs are also usually consistent for a given animal. Amaurosis (central blindness) is common before and after seizures and is frequently noted by owners. • The condition should be suspected in any Arabian foal showing coat color dilution with appropriate clinical signs. It may be somewhat more difficult in foals without obvious coat color dilution. • There is genetic testing available in the US and Australia at present. • There is no effective treatment and affected foals are usually euthanized within a few days of birth. • The brainstem auditory-evoked response (BAER) test measures responses in brain waves that are stimulated by clicks in the ear to check the auditory pathways of the brainstem. • Normal values have not been established for foals, however marked delays can be predicted from normal adult horse values. • HIE is principally a diagnosis of exclusion. Foals typically present within 24–36 hours of birth. • The condition should be expected in any foal which has a history of abnormal placentation of the mare, premature placental separation, delivery by cesarian section, prolonged dystocia, premature delivery or in foals that have required resuscitation for any reason after delivery. • Treatment of the mare. Prevention of intrauterine asphyxia by early treatment of placentitis in mares may help to decrease intrauterine asphyxia (see Chapter 12, p. 471). • Maintaining adequate ventilation • Maintaining adequate perfusion • Maintaining adequate glucose levels • Control of brain swelling (see Treatment of CNS trauma in p. 410) 1. Free radical scavengers: Allopurinol (xanthine oxidase inhibitor) 2. NMDA (N-methyl-D-asparate) receptor blockers such as magnesium 4. Thiamine: 1 gram IV in 1L of fluids SID. Thiamine increases the activity of the ATP-dependent sodium pump, thus regulating ion uptake and decreasing cellular water 5. Hypothermia has been shown to be neuroprotective. However the exact level of hypothermia has not been established for equine patients but currently it is advisable to stay below rather than above normal temperature. Thus avoid overheating by the judicious use of heating lamps, blankets, etc. • Survival rates have been reported as high as 90% but the prognosis is poorer for premature foals, foals with concurrent sepsis and foals showing clinical signs immediately after birth. There are no abnormalities between episodes and cardiovascular function is normal. • The diagnosis is made on clinical signs. Electroencephalography is used in other species, but interpretation in foals requires considerable experience. • Differential diagnosis: Epilepsy and cardiovascular causes of collapse (these however are not associated with rapid eye movement or loss of reflexes). Cervical vertebral malformation (CVM) is a common cause of ataxia in horses and tends to affect young adults. Compression of the cervical spinal cord results in lesions to proprioceptive and motor tracts to the thoracic and pelvic limbs. This results in ataxia (resulting in inconsistent foot placement and excessive circumduction of the pelvic limbs when turning) and paresis (shown by weakness when pulling on the tail while the horse is walking in a straight line). Table 11.1 outlines a system for grading neurological deficits that is useful in the evaluation of horses with suspected CVM as it allows for accurate recording and monitoring of progression. There are two different types of CVM/CVSM that are recognized and are outlined in Box 11.1. Table 11.1 System for grading of neurological gait deficits • Neurological examination usually reveals symmetrical ataxia, paresis, dysmetria and spasticity in all four limbs, though usually more noticeable in the pelvic limbs. Asymmetry of clinical signs can be seen in horses with significant degenerative joint disease of the articular processes. At a walk signs of ataxia and paresis such as truncal sway, circumduction of the hindlimbs, toe-dragging and stumbling can be seen. These signs can be exacerbated by walking the horse up or down a slight slope, walking over obstacles (e.g. kerb), turning in circles or elevating the horse’s head. Evidence of hypermetria such as exaggerated limb movements or hypometria such as stiff-legged movements are also frequently seen. • The diagnosis can be made from good-quality cervical radiographs. Subjective assessment reveals enlarged physeal growth plates, caudal extension of the dorsal border of the orifice of the vertebral canal, angular fixation, delayed ossification of bone and degenerative joint disease. • Stenosis of the vertebral canal corrected for radiographic magnification can be determined by measurement of the intravertebral ratio. A ratio of less than 50% at C4, C5 or C6 or less than 52% at C7 is associated with a high likelihood of having CVSM. • Myelography can be used to confirm stenosis of the vertebral canal but should only be used when the results will alter the treatment of the case. • Contrast-enhanced computed tomography has been used in a few cases. Currently the indications are presurgical evaluation and assessment of lateral compressive lesions in horses without myelographic evidence of compression that are strongly suspected of having lesions based on clinical signs. Availability, cost and patient size are limiting factors for the use of this technique at present. • Early diagnosis of CVM in young Thoroughbred horses has been successfully treated using a restricted, paced diet and confinement. Treatment with anti-inflammatory drugs is also common. The use of corticosteroids is controversial due to lack of evidence regarding efficacy, potential negative side effects and the fact that corticosteroids are contraindicated in EPM. Without definitive diagnostic techniques differentiation between CVM and EPM in endemic areas may be difficult. • Although fusion of cervical vertebrae can result in resolution of clinical signs in selected adult cases of CVM, it would be hard to justify in a foal. These fractures or their central nuclei will have dramatic and easily recognized signs (see Fig. 11.20). In the former, blindness and pupil-light-reflex deficiencies or discrepancies between the two eyes will be present. Concurrent retinal damage (including detachment) and/or lens dislocation may be found. • In many cases the diagnosis of trauma is an obvious diagnosis but diagnostic imaging techniques can be useful to help identify the exact site and thus allow for a more informed assessment of involved structures. Advanced imaging techniques such as CT and MRI can provide additional information such as enlargement of cerebral hemispheres. • Basioccipital and basisphenoid fractures may be difficult to appreciate radiographically, but endoscopic examination of the roof of the medial compartment of the guttural pouches will often identify either bruising or even the presence of a hematoma over the site of the fracture. • Fractures of the hyoid bone may possibly be detected radiographically and by endoscopic examination of the guttural pouch(es). • Ultrasound examination of the atlanto-occipital space can be used in some cases to determine the presence of intracranial hemorrhage. • Cisternal puncture and aspiration of blood-stained cerebrospinal fluid confirms intracranial hemorrhage but in many cases may be contraindicated due to increased risk of mid brain herniation. • The general principles for treatment of CNS trauma are administration of osmotic diuretics, nutritional and fluid support and protection from self-inflicted trauma and the effects of prolonged recumbency. • Seizures or excessive, difficult-to-manage thrashing may require sedation or short-term anesthesia. Diazepam 5 mg (foal) up to 100 mg (adult) can be repeated as necessary to control seizures. Phenobarbital or pentobarbital may also be used to control seizures. The alpha 2 agonists should be avoided for the treatment of seizures in the acute stages as they can cause transient hypertension, exacerbating CNS hemorrhage and they also suppress ventilation. • Horses with CNS signs following cranial trauma should probably receive dexamethasone (0.1–0.25 mg/kg q 4–6 h) for 1–4 days. However, the benefits should be weighed against the possible complications of steroid administration in the horse (laminitis). • Intravenous administration of 20% mannitol (0.25–1 g/kg) has been used for the treatment of increased intracranial pressure. Its use however is contraindicated in cases of ongoing cerebral hemorrhage. Response to mannitol is usually noted within 1 hour and if a response is noted, mannitol administration should be repeated every 4–6 hours for the first day. • DMSO, is frequently given slowly at 1 g/kg as a 10% solution in isotonic fluids. DMSO has several beneficial pharmacological effects including diuresis, free-radical scavenging, inhibition of platelet aggregation, vasodilation and increased penetrance of steroids and antibiotics into the brain. There are many anecdotal reports of successful treatment with DMSO but clinical trials in other species have not shown a clear benefit in treating CNS trauma. Adverse effects include intravascular hemolysis which has been associated with too rapid administration or administration of a more concentrated solution. DMSO administration can be repeated every 12 hours for 3–4 days if clinical improvement is seen. • Hypoxemia and hypercapnia should be avoided as hypoxia exacerbates brain swelling and hypercapnia increases intracranial blood volume and pressure. Recumbent horses should be rolled every 4–6 hours to minimize pulmonary arteriovenous shunting and ventilation perfusion mismatching. Ideally the head should also be maintained at heartbase level or higher to avoid hypostatic intracranial congestion. Maintenance of hydration is important but over-hydration should be avoided as it can exacerbate brain edema. • Close monitoring and good nursing care are essential. If an improvement is noted in 6–8 hours, treatment should be repeated. If no improvement is seen more aggressive treatment may be warranted, including exploratory craniotomy. • Complicated combinations of neurological deficits can arise from even relatively minor trauma and may take some hours or days to produce their full neurological effect. Many consequences of cranial trauma such as hemorrhage, laceration necrosis, secondary ischemia and midbrain injury are inaccessible to therapy and the presence of some of these lesions is difficult to diagnose with failure to improve or deterioration in neurological condition being the only clue to their existence. • The most accurate prognosis is based on repeated detailed neurological examinations with assessment of the rate of progression or resolution and the responses to specific therapeutic measures. • Cerebral and cerebellar lesions usually carry a better prognosis than brainstem lesions and therefore those injuries which are accompanied by bleeding from the ear but few other outward signs are possibly more serious than the more dramatic injuries to the frontal and facial bones. • If no improvement is seen or deterioration is noted in a comatose patient 36 hours after surgery or anesthesia, euthanasia may be indicated. • Plain radiography is the most helpful aid in confirming vertebral trauma, but does not directly evaluate the presence or extent of spinal cord damage. Abnormalities that are seen that indicate injury are displacements of vertebral components, shortened or abnormally shaped vertebrae, slipped physeal plates and fractures. • Fracture-induced changes in the CSF may be useful for ancillary diagnosis. These changes can be classified as acute (<24 hours) or chronic (>24 hours). The acute changes include diffuse blood contamination, a high red blood cell (RBC) count, a normal to high white blood cell (WBC) count, and a high protein concentration. Chronic CSF changes include a normal to slightly increased WBC count, normal to increased RBC count, increased protein concentration and xanthochromia. • Pain should be managed with non-steroidal anti-inflammatories and other medication recommendations are similar to those for cerebral injury. Good nursing care is essential, especially for recumbent patients and should include bladder and rectal evacuation if necessary. • If the spinal fracture appears stable and the animal can stand with assistance, it may be placed in a water tank and supported for long periods. Other methods of support include slings, but these should not be used for animals that cannot support themselves as severe respiratory compromise or myositis may result. Slinging of animals with mild neurological signs may help minimize secondary complications, improve extensor tone and hasten recovery. • Horses which suffer from spinal pain and/or posterior paralysis often show extremes of panic and their management is most difficult and frequently dangerous for the handlers involved. Priority should be given to human safety at all times. • The prognosis is best judged on the basis of repeated neurological examinations. The longer a patient remains recumbent and neurologically impaired the poorer the prognosis. In general the prognosis for horses suffering from spinal trauma is poor. Some may recover with time but the intensity of nursing required and the consequences of incoordinated attempts to rise are often most distressing for the horse, the owner and the attendants. • Healing of fractures frequently results in vertebral malalignment. Delayed callus formation and degenerative changes in adjacent articulations can result in permanent spinal cord compression even after apparent healing and resolution of clinical signs.
Disorders of the nervous system
Developmental disorders
Hydrocephalus (Figs. 11.1–11.3)
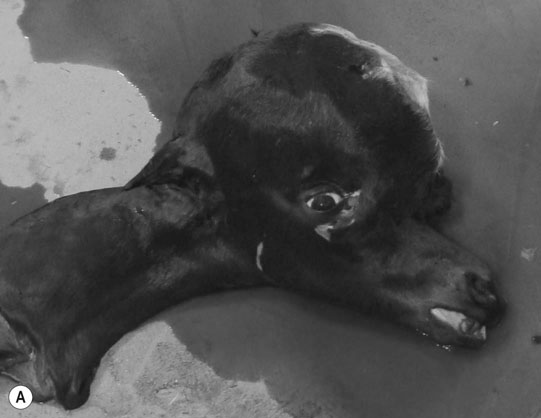
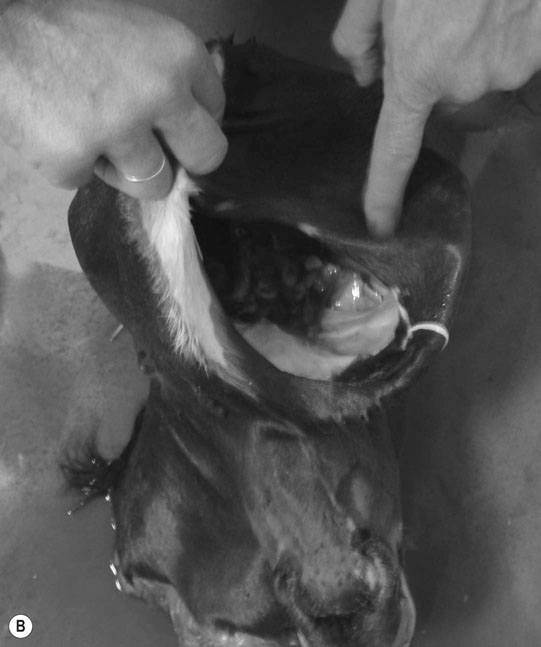

Differential diagnosis: Any condition capable of producing abnormal behavior patterns including HIE, electrolyte imbalances/hypoglycemia, meningitis/septicemia and prematurity.
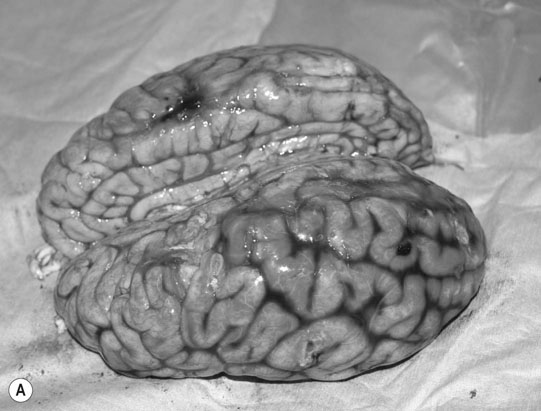
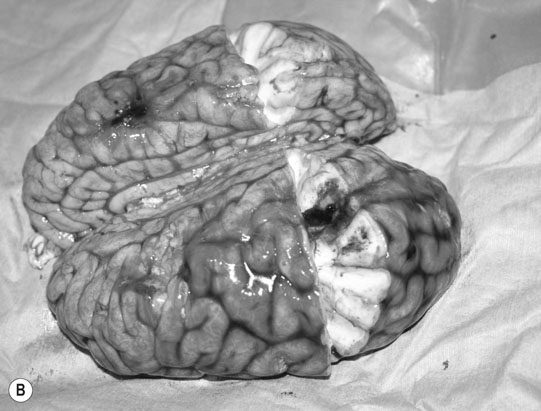
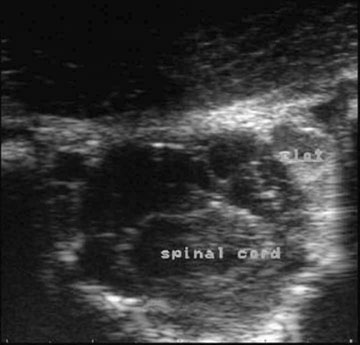
Cerebellar hypoplasia (Fig. 11.6)
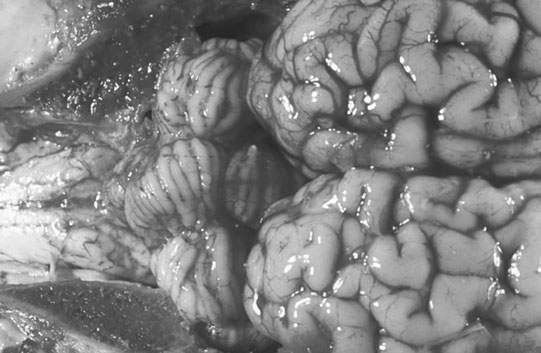
Diagnosis and treatment
Cerebellar abiotrophy
Diagnosis and treatment
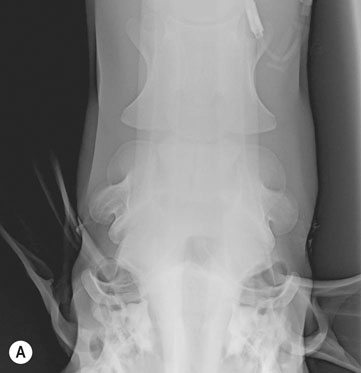
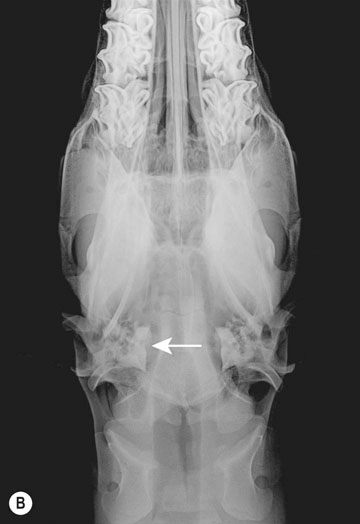
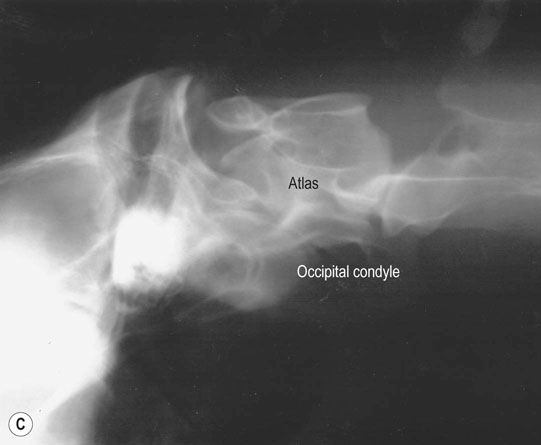
Occipitoatlantoaxial malformation (OAAM) (Fig. 11.7)
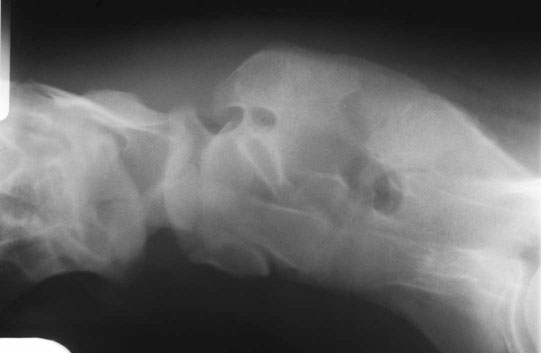
Differential diagnosis: Cervical fractures and cervical vertebral malformation should be ruled out.
Diagnosis and treatment
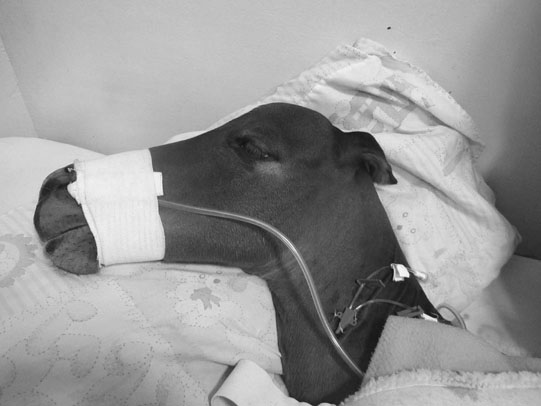
Juvenile epilepsy (Fig. 11.8)

Differential diagnosis: (any condition capable of producing seizure activity)
Clinical signs
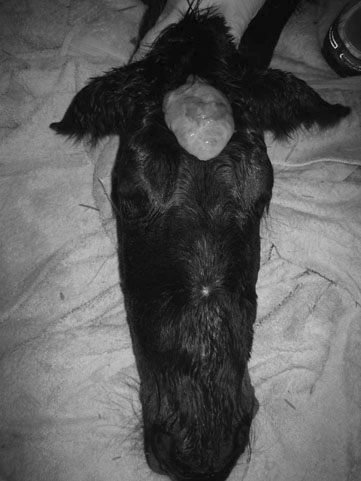
Lavender foal syndrome/coat color dilution lethal (Fig. 11.9)
Diagnosis and treatment
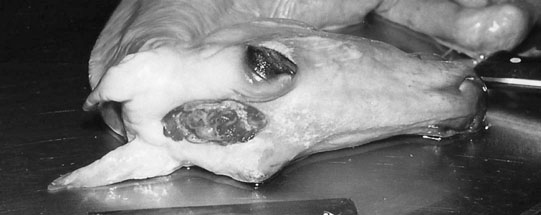
Deafness (Fig. 11.10)

Diagnosis and treatment
Hypoxic ischemic encephalopathy (neonatal maladjustment syndrome) (Fig. 11.11)


Differential diagnosis:
Diagnosis
Treatment and prognosis
Narcolepsy/cataplexy
Diagnosis and treatment
Non-infectious disorders
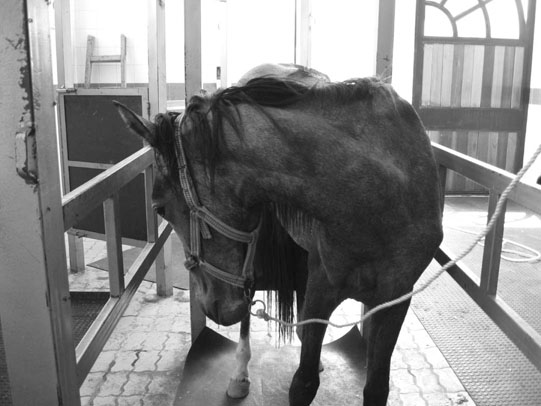
Cervical vertebral malformation/cervical vertebral stenotic myelopathy (Figs. 11.12–11.15)
Grade
Description
0
No gait deficit
1
Deficit barely detectable at walk or trot, but present with special tests
2
Deficit detected during walk and trot, exaggerated by special tests
3
Deficit prominent at walk or trot, may fall during special tests
4
Stumbling, tripping or falling spontaneously at normal gait
5
Down, cannot rise


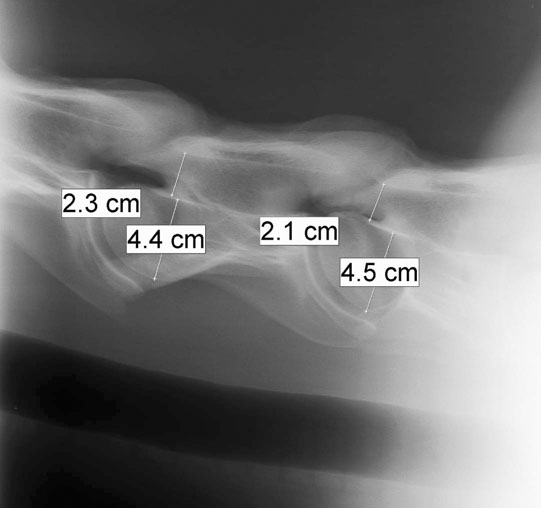
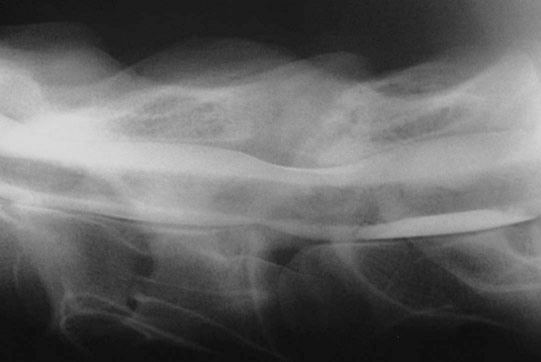
Diagnosis
Treatment
Traumatic injuries
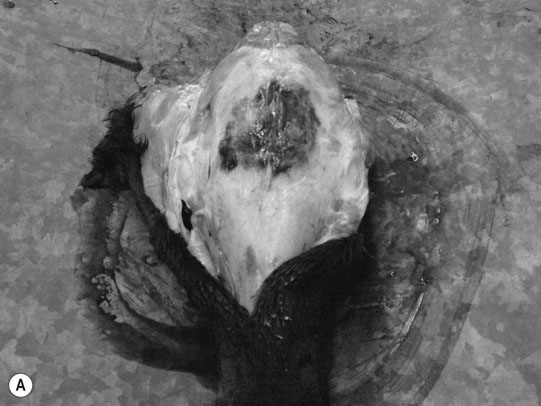
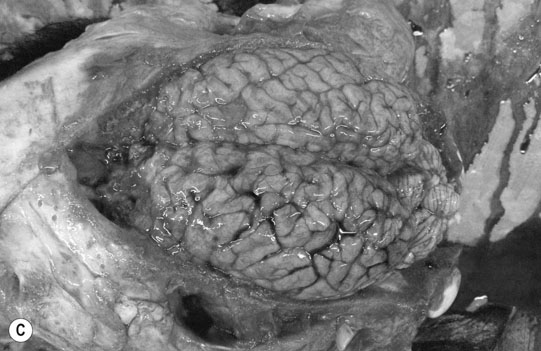
Cerebral trauma (Figs. 11.16–11.20)




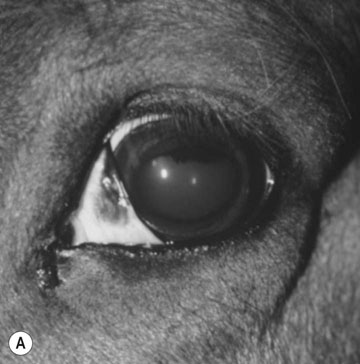
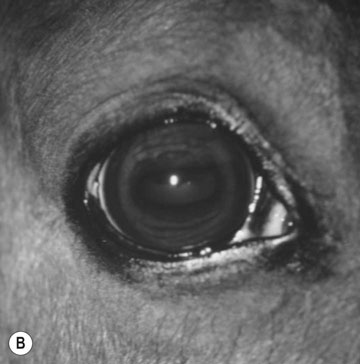
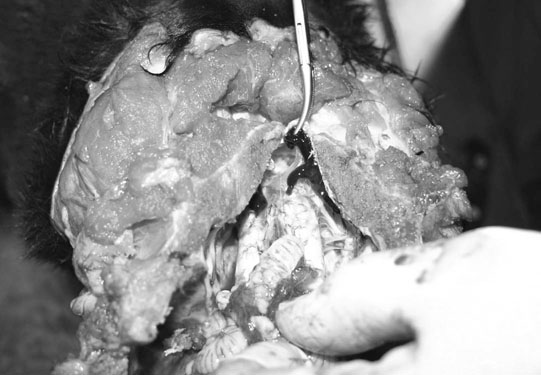
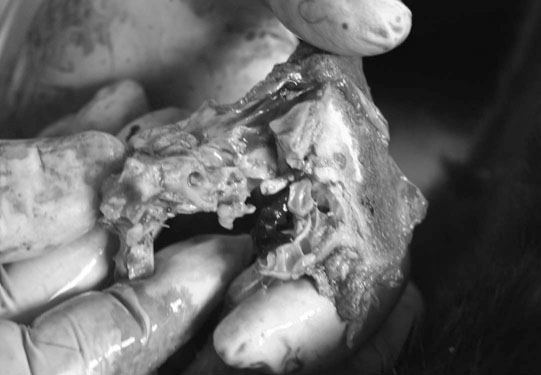
Basioccipital and basisphenoid fractures (Figs. 11.21 & 11.22)

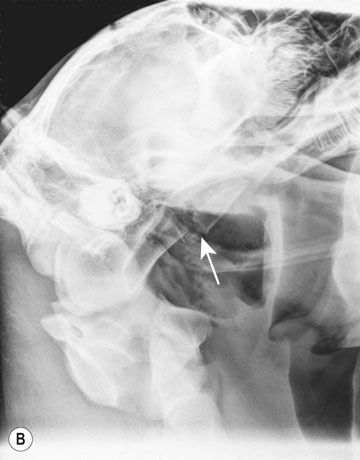
Differential diagnosis:
Petrous temporal bone fractures (Figs. 11.23–11.30)
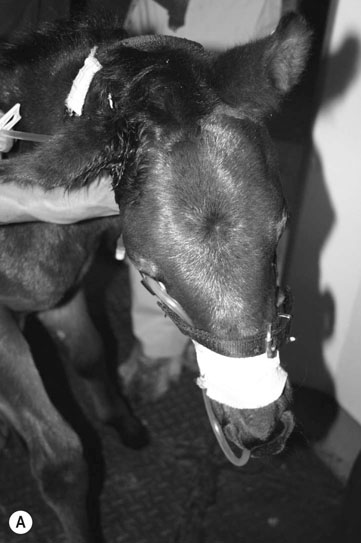
Fractures which affect the optic or vestibulo-cochlear nerves
Diagnosis
Treatment
Prognosis
Cord trauma
Cervical spine (Figs. 11.31–11.34)

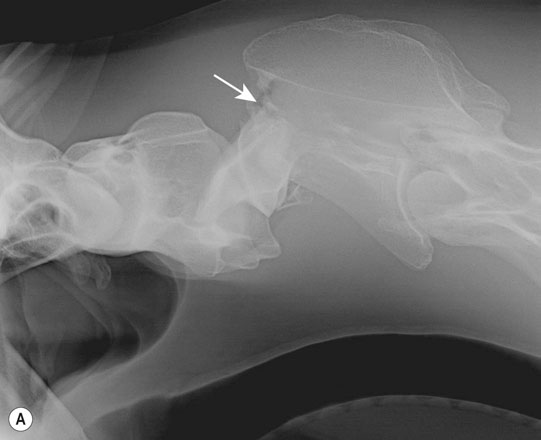
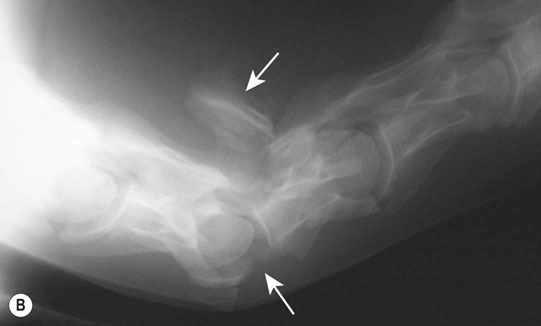
Thoracic spine (Figs. 11.35–11.38)


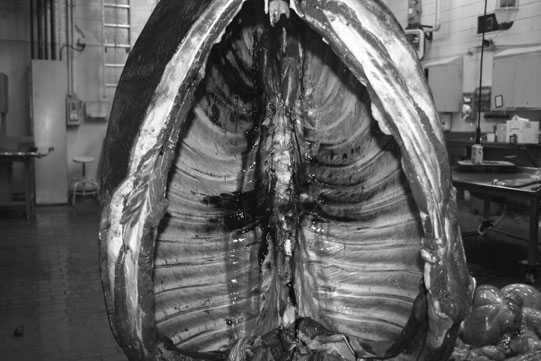


Lumbar spine (Figs. 11.39–11.41)




Sacrococcygeal spine
Clinical pathology and radiographic findings
Treatment
Prognosis
Other cranial nerve disorders
The trigeminal nerve (CN-V) (Fig. 11.42)

Differential diagnosis:

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Disorders of the nervous system