Chapter 10 Cornea and Sclera
ANATOMY, PHYSIOLOGY, AND WOUND HEALING
Cornea
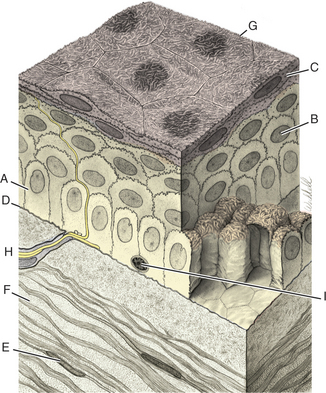
The cornea has the following four layers (Figure 10-1):
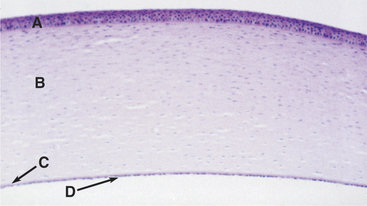
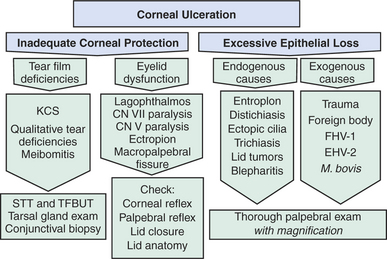
Figure 10-1 Photomicrograph of the feline cornea. A, Epithelium; B, stroma; C, Descemet’s membrane; D, endothelium.
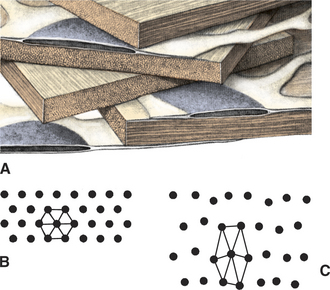
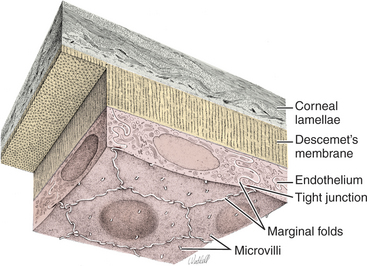
The corneal epithelium is stratified, squamous, and nonkeratinized. From deep to superficial it comprises the basement membrane, basal epithelial cells, wing cells, and squamous surface cells (Figure 10-2). Basal cells are attached to the basement membrane by hemidesmosomes. As basal cells divide, daughter cells are forced toward the surface, become flattened as wing cells, and gradually lose many of their organelles. Basal cells are replaced by stem cells at the limbus that are constantly undergoing mitosis and migrating centripetally. Surface squamous cells possess microvillous projections that anchor the deep mucin layer of the precorneal tear film. The corneal stroma, composed of keratocytes, collagen, and ground substance, constitutes 90% of the corneal thickness and lends rigidity to the globe. The parallel collagen fibrils form lamellae of interlacing sheets (Figure 10-3), with occasional interspersed keratocytes (which are modified fibroblasts), lymphocytes, macrophages, and neutrophils. The regular spacing of stromal collagen fibrils maintains corneal transparency and distinguishes corneal stroma from the collagen in scar tissue and sclera.
Descemet’s membrane is the basement membrane of the endothelium, lying between the posterior stroma and the endothelium (see Figures 10-1 and 10-4). Because it is continuously secreted by endothelial cells throughout life, this membrane thickens with age. It is very elastic but can break from globe stretching (buphthalmos), as seen with advanced glaucoma, or with penetrating injuries or ruptured ulcers. Descemet’s membrane becomes exposed in ulcers in which there is complete stromal loss (descemetoceles). It does not stain with fluorescein and therefore appears as a dark, transparent, sometimes outwardly bulging structure in the center of a deep corneal ulcer or wound.
Factors that alter the collagen lattice or spacing of collagen fibrils, the optical surface, or the type of collagen all reduce corneal transparency (see Figure 10-3). Common examples are corneal edema, corneal scarring, loss of epithelium, alterations in the precorneal tear film, elevated intraocular pressure (IOP), damage to endothelium, formation of scar tissue, altered glycosaminoglycan content, melanosis, corneal vascularization, and infiltration of the stroma with white blood cells.
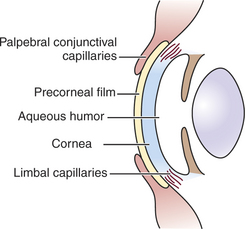
Because the cornea is avascular, oxygen and nutrients must be obtained and metabolites disposed of through alternate routes (Figure 10-5). Such routes include the aqueous humor, the precorneal tear film and the atmosphere, and adjacent capillary beds in the sclera and bulbar and palpebral conjunctiva. The endothelium and posterior stroma receive most of their nutrients from the aqueous humor, but the tear film and atmospheric oxygen are the major sources for the anterior cornea.
Normal Corneal Healing
Stroma
Avascular healing of corneal stroma occurs as follows:
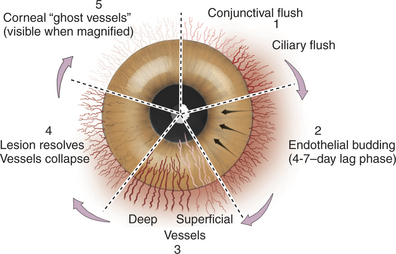
In vascularized healing of destructive lesions, cellular infiltration is more extensive, and the area is invaded by blood vessels originating from the limbus (Figure 10-6). Granulation tissue is laid down and forms a denser scar than in avascular healing. Eventually the blood vessels cease to be perfused, but they remain as “ghost vessels” and are visible on slit-lamp examination. If inflammation recurs at a later date, these ghost vessels may rapidly become perfused, creating the clinical appearance of more severe or chronic inflammation than is actually present. Corneal nerves damaged by the lesion gradually regenerate, and sensation returns slowly to the affected area.
Effects of Corticosteroids on Corneal Healing
Topical corticosteroids limit corneal opacification by inhibiting fibroplasia, decreasing vascularization, and reducing melanosis. They also control the potentially blinding consequences of anterior uveitis that frequently accompanies corneal wounds. However, corticosteroids also inhibit epithelial regeneration, corneal infiltration with inflammatory cells, fibroblastic activity, and endothelial regeneration. The strength of the resulting wound is lessened, collagenases are potentiated up to 15 times, and the risk of infection is greatly enhanced when corticosteroids are used. These potential negative and positive effects on wound outcome and healing must be considered carefully. Typically there is good justification for the use of topical corticosteroids provided that:
Sclera
Numerous channels exist in the sclera through which vessels and nerves pass. These channels also provide routes by which disease processes such as infections and neoplasia may enter or leave the eye. The optic nerve leaves the eye through a sievelike perforation of the sclera at the posterior pole called the lamina cribrosa. Alterations in tension on the lamina cribrosa during glaucoma reduce the size of the spaces through which nerve axons pass and interfere with axoplasmic flow within the optic nerve, thereby contributing to optic nerve degeneration in this disease. The short posterior ciliary arteries and nerves pierce the sclera around the optic nerve and enter the choroid. The long posterior ciliary arteries and nerves pierce the sclera near the optic nerve and pass anteriorly around the eye to the ciliary body. Anterior ciliary arteries and vortex veins enter and leave the sclera in the area overlying the ciliary body. The intrascleral venous plexus lies anteriorly in the outer portion of the scleral stroma. It receives aqueous humor from the area of the iridocorneal angle via the angular aqueous plexus and aqueous veins and then passes the aqueous humor to the choroidal venous system (see Chapter 12).
In fish, lizards, birds, and some amphibians, cartilaginous and/or bony ossicles may form part of the sclera. These structures are thought to enhance ocular rigidity and aid in accommodation. Surgical techniques for removal of the eye (enucleation) must allow for these ossicles (see Chapter 20).
PATHOLOGIC RESPONSES
Corneal disorders are of three general categories with respect to origin, as follows:
Exogenous insults must first pass through or damage the corneal epithelium, which—despite its special properties and precarious position—is an extremely effective barrier to most microbial organisms. With the exception of Moraxella bovis and the herpesviruses, microorganisms cannot initiate primary keratitis in animals. However, once the epithelium has been breached, most microorganisms can readily establish themselves and spread within the avascular stroma. The efficiency of the epithelial barrier is indicated by the frequency with which pathogenic bacteria are found in the normal conjunctival sac of domestic animals but the relative rarity of primary disease arising from their presence (see Chapter 3).
Corneal Reactions to Disease
Corneal Edema
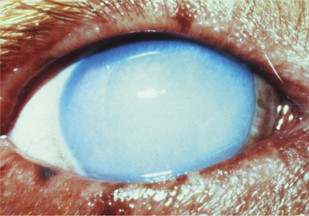
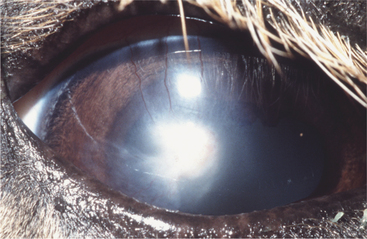
Control of entry of water into the cornea and maintenance of a state of relative dehydration is critical to corneal transparency. Corneal edema results when excess fluid accumulates within the stroma and forces the collagen lamellae apart, leading to loss of transparency (Figure 10-7). The endothelium makes the major contribution to control corneal stromal fluid balance by moving solutes (and therefore water) from the stroma to the aqueous against the IOP gradient that forces water into the cornea. The epithelium plays a lesser but critical role by preventing tears from entering the stroma. Dysfunction of either of these cell layers leads to stromal edema and loss of transparency. For example, if the corneal epithelium becomes ulcerated, water enters the stroma from the precorneal film, and gross swelling and discoloration occur until a new layer of epithelium has covered the area and fluid balance is restored. Endothelial cell loss tends to cause more marked and more diffuse corneal edema. Because endothelial cells are postmitotic and do not regenerate, some degree of endothelial cell loss and dysfunction occur as part of the normal aging process in all domestic species. This physiologic loss of endothelium does not cause appreciable edema. Hastened cell loss may also occur as a result of primary corneal or intraocular disease processes such as corneal endothelial dystrophy, glaucoma, uveitis, and anterior lens luxation. Regardless of cause, corneal edema appears hazy blue and may be localized or diffuse. Corneal edema is reversible if the underlying cause is removed, sufficient endothelial cell function remains, and fluid balance is reestablished. Severe corneal edema may result in epithelial bullae formation (bullous keratopathy) and sometimes corneal vascularization (see later).
Corneal Vascularization
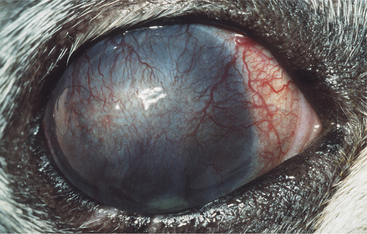
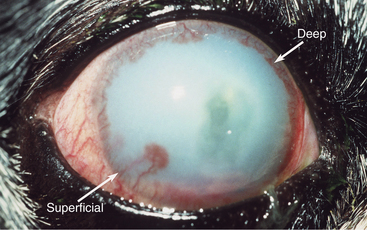
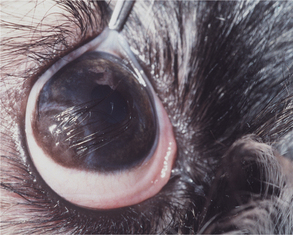
The normal cornea contains no blood vessels; however, vessels invade the corneal stroma in response to various pathologic processes and especially during vascularized stromal healing. Corneal vascularization may be superficial, deep, or both. Superficial vessels occur in the anterior third of the stroma and appear “treelike”; that is, they usually begin at the limbus as a single vessel and branch extensively within the cornea (Figure 10-8). Very superficial vessels may be seen crossing the limbus because they are continuous with the conjunctival circulation. Deep intrastromal vessels appear more “hedgelike”; that is, they are shorter and straighter, branch less, and look like paintbrush strokes (Figure 10-9). They appear to arise from under the limbus because they are continuous with the ciliary circulation. The depth of the invading vessels is usually a very accurate indication of the depth of the initiating lesion—deep vessels suggest corneal stromal or intraocular disease, whereas superficial vessels are induced by surface (usually corneal epithelial) disease. The sequence of vascularization is shown in Figure 10-6. In complicated and persistent corneal lesions, aggressive vascularization with granulation tissue formation occurs.
Corneal Fibrosis
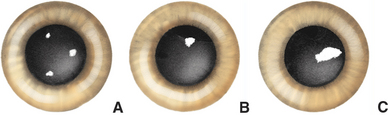
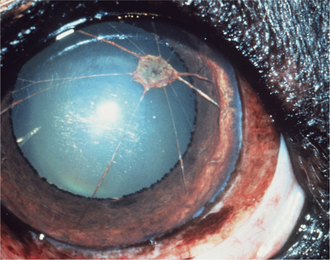
Collagen fibrils produced during repair of a stromal lesion are not laid down in a regular lattice pattern and so interfere with light transmission. This produces a grey “wispy” or “feathery” opacity within the cornea under a normal epithelium that is not associated with other signs of inflammation except perhaps some residual corneal blood vessels (Figure 10-10). With time, scars may clear optically but often do not do so completely. The tendency to clear is greater in young animals and also in cattle, sheep, and cats. Melanosis of the scarred area often occurs in dogs. In dogs lipid deposition may also occur near the scar. The deeper the initial injury, the more dense and permanent the scar and the lesser the tendency for transparency to return. With increasing size, a corneal scar is termed a nebula, macula, and leukoma (Figure 10-11). If the iris attaches to the posterior surface of the leukoma owing to anterior synechia, the lesion is called an adherent leukoma.
Corneal Melanosis
Corneal melanosis is frequently called corneal pigmentation or pigmentary keratitis (Figure 10-12). This term ignores the fact that pigments other than melanin (such as hemoglobin or the soluble pigment in corneal sequestra of cats) may cause opacification of the cornea; therefore corneal melanosis is the preferred term. Regardless of the term chosen, it is often used as if it were a clinical diagnosis; actually, corneal melanosis is merely a sign of chronic corneal irritation that may arise from any number of causes, each with a different treatment and prognosis. Melanin is deposited in the corneal epithelium and sometimes the anterior stroma and originates from proliferation and migration of normal limbal melanocytes during corneal inflammation. The more heavily melanotic the limbus, the more likely and the denser the corneal melanosis.
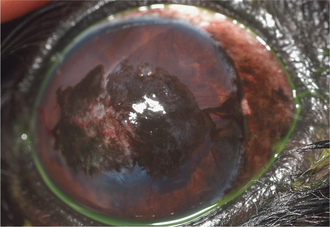
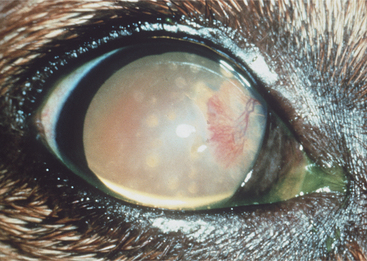
Figure 10-12 Corneal melanosis and vascularization due to brachycephalic ocular syndrome in a shih tzu.
(Courtesy University of California, Davis, Veterinary Ophthalmology Service Collection.)
At the very least, animals with corneal melanosis should undergo the following evaluations:
Stromal Infiltration with White Blood Cells
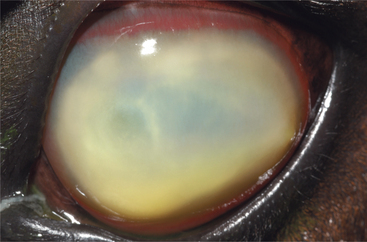
Inflammatory cell infiltration of the corneal stroma appears as yellowish green discoloration (Figure 10-13). It usually occurs in response to an infection or in association with a corneal foreign body. However, nonseptic infiltration of the cornea can occur. This finding is most dramatic in the equine cornea, is commonly seen in dogs, and is relatively infrequently seen in cats. Inflammatory cells originate from the tear film, limbus, or uveal tract (via the aqueous humor) and can accumulate within the corneal stroma surprisingly quickly, indicating a potent chemotactic stimulus. Corneal cytology along with culture and sensitivity testing should be performed, and broad-spectrum antibiotic therapy initiated promptly. Frequent reexamination of the cornea is justified because liberation of lytic enzymes from inflammatory cells, microbes, and corneal cells can be associated with rapid collagenolysis (i.e., malacia or corneal “melting”).
Accumulation of Abnormal Substances (Lipid or Mineral) within the Cornea
Lipid and/or mineral accumulation appears as sparkly, crystalline, or shiny white areas in the cornea. These accumulations frequently contain cholesterol and/or calcium in varying combinations. All corneal layers may be involved, but lipid/mineral deposits are usually subepithelial, so the cornea does not retain fluorescein stain. Such accumulation occurs as a primary or inherited, but not necessarily congenital, condition in many canine breeds (corneal lipid dystrophy) but rarely in other species. It may also occur as an acquired, inflammatory condition (corneal lipid degeneration) in dogs and horses but rarely in cats. Lipid dystrophy is typically bilateral and nonpainful, has minimal effect on vision, and requires no therapy (Figure 10-14). Corneal lipid degeneration, by contrast, is usually unilateral and frequently associated with inflammation (keratitis, scleritis, or uveitis). Coincident corneal edema, vascularization, fibrosis, and melanosis are common (Figure 10-15). Occasionally there is a history of ocular trauma, often with ulceration that healed with lipid deposition. Corneal lipid accumulation may also occasionally be seen with long-term corticosteroid use.
Figure 10-14 Corneal lipid/mineral dystrophy in a dog.
(Courtesy University of California, Davis, Veterinary Ophthalmology Service Collection.)
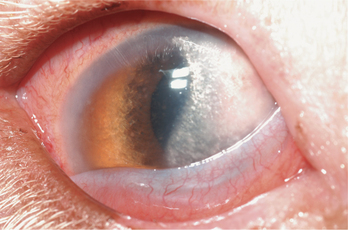
Figure 10-15 Corneal lipid degeneration and edema in a dog with episcleritis.
(Courtesy University of California, Davis, Veterinary Ophthalmology Service Collection.)
In some animals, deposition of lipid in the cornea is due to elevated serum lipid concentrations. Investigation of common causes of systemic hyperlipidemia, such as hypothyroidism, diabetes mellitus, hyperadrenocorticism, and primary hyperlipidemia, is therefore warranted. Both serum cholesterol and triglyceride concentrations should be assessed. If serum lipid values are elevated, dietary management and treatment for the underlying cause are necessary. Surgical removal of lipid plaques is contraindicated until hyperlipidemia is corrected, because keratitis from the keratectomy increases lipid deposition during healing. A specific lipid keratopathy is noted in frogs (see Chapter 20).
Stromal Malacia (or “Melting”)
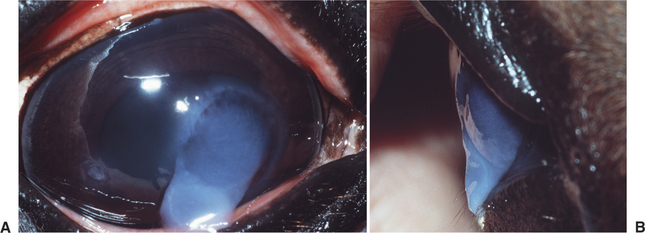
Stromal malacia or “melting” occurs as a result of collagenolysis due to collagenase liberation from invading white blood cells, especially neutrophils within the corneal stroma, microorganisms, or corneal epithelial cells or keratocytes. The result is loss of rigidity and structure of the corneal collagen with subsequent “sagging” or “oozing” of the stroma over the ventral cornea or eyelid (Figure 10-16), followed by stromal loss with development of a deep ulcer or descemetocele. This change can occur very quickly and in relative isolation if the stimulus for collagenase production is marked and rapid but is more commonly seen in association with other corneal pathology, notably stromal white cell infiltration and edema.
CORNEAL DISEASES BELIEVED TO BE INHERITED
Microcornea
Microcornea can be diagnosed through measurement of the horizontal and vertical diameters of the cornea and comparison of the results with values from the other eye (Figure 10-17). The condition is usually unilateral and associated with microphthalmia (see Chapter 2).
Dermoid
Dermoids are examples of a choristoma or congenital circumscribed overgrowth of microscopically normal tissue in an abnormal place (Figure 10-18). They may involve conjunctiva, third eyelid, eyelid margin, limbus, or cornea in various combinations. In dermoids containing hair follicles, hair grows from the surface, which causes conjunctival and corneal irritation evident as corneal opacity, conjunctival hyperemia, and ocular discharge. Dermoids usually grow slowly, if at all. They are believed to be inherited in Hereford cattle, Birman and Burmese cats, Saint Bernard dogs, dachshunds, and Dalmatians. Treatment requires careful surgical excision with conjunctivectomy, combined with keratectomy if they cross the limbus. Because the cornea underlying dermoids may be thinner than normal, referral to an ophthalmologist is recommended.
Persistent Pupillary Membranes
Persistent pupillary membranes (PPMs) are an inherited failure of the uveal tract to regress appropriately during embryologic and immediate postnatal development. They are more fully discussed in Chapter 11. However, they can cause corneal opacity if they arc from the iris collarette to the corneal endothelium (Figure 10-19), where they are associated with corneal edema, melanosis, and/or fibrosis. These are noninflammatory and vary in their effect on vision according to severity. No therapy is possible or necessary for PPMs; however, affected animals should not be bred.
Superficial Punctate Keratitis
The term superficial punctate keratitis is applied to multiple, superficial, circular defects in the corneal epithelium that may or may not stain with fluorescein (Figure 10-20). The affected areas are scattered diffusely across the corneal surface, sometimes leaving the cornea looking like the skin of an orange. The condition is recognized as familial in shelties and dachshunds, may be due to a qualitative tear film deficiency (most likely mucin deficiency), and often responds to cyclosporine applied topically twice daily. Recurrences are frequent. Similar (nonrecurrent and noninherited) keratopathies may be induced by mild corneal insults, such as exposure during general anesthesia and use of topical anesthetics.
Corneal Lipid Dystrophy
Deposition of lipid or, occasionally, mineral in the anterior stroma (immediately subjacent to the epithelium) is relatively common in dogs and is seen rarely in cats and horses. Deposits are usually central or isolated from the limbus and are bilateral although they may be asymmetrical (see Figure 10-14). The condition appears to be familial and may be static or slowly progressive. The lipid may take a variety of forms, including circles, ovals, and arcs concentric with the limbus. This condition can be differentiated from corneal degeneration (see Figure 10-15) or diseases caused by circulating hyperlipidemia, because corneal lipid dystrophy is noninflammatory, lacks connection with the limbus, and is associated with normal fasting serum cholesterol and triglyceride concentrations.
ACQUIRED CORNEAL DISEASES
Corneal Ulcers
Optimal management of corneal ulcers requires knowledge of the following information:
Common Causes of Corneal Ulceration
Corneal epithelium is constantly being physiologically abraded by normal blinking and desiccation and being replaced by normal cell turnover. Rate of regeneration and surface protective mechanisms are usually sufficient to ensure that ulceration does not occur. Therefore, from a purely mechanistic viewpoint, corneal ulcers may be thought of as arising when this situation becomes unbalanced owing to decreased corneal epithelial protection or increased corneal abrasion (Figure 10-21). Corneal protection is provided by the tear film along with the upper, lower, and third eyelids. Inadequate production, retention, or dispersion of the tear film is therefore frequently associated with corneal ulceration.
Excessive abrasion can be further divided into endogenous causes, such as abnormal lid position or anatomy and eyelash abnormalities, and exogenous causes, such as trauma and foreign body retention in the conjunctival fornix—all of which are common in dogs but less common in most cats because of breeding practices and the relatively more protected environment in which cats tend to live. Brachycephalic cats are more likely to have these eyelid/cilia abnormalities. Horses are susceptible to exogenous trauma but rarely suffer eyelid or tear film abnormalities. Infectious agents also qualify as exogenous causes of corneal ulceration, but it should be noted that other than M. bovis and the herpesviruses, primary infectious ulceration does not occur.
Simple versus Complicated Ulcers
Nonhealing ulcers in all patients can be assigned to one of the following three categories:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree