Chapter 3 Cardiovascular Diseases
EXAMINATION OF THE CARDIOVASCULAR SYSTEM
Evaluation of the jugular veins for pulsation and distention requires differentiation of the “notorious” false-jugular pulsation commonly observed in thin-necked dairy cattle from pathologic true jugular pulsation and distention. False or normal jugular pulsation is a product of reverse blood flow from atrial contraction at the end of diastole and expansion of the right atrioventricular (AV) valve during systole. Passive jugular filling during systole also may contribute, as does a “kick,” or referred carotid artery pulsation. False jugular pulsation arises as a wave that winds its way from the thoracic inlet to the mandible when the cow has her head and neck parallel to the ground. When the head and neck are raised, the false jugular pulse may only ascend a portion of the cervical area or may disappear. A true jugular pulse fills the whole jugular vein rapidly when the head and neck are parallel to the ground or slightly raised. This rapid filling is similar to filling a garden hose with the end held off when water to the hose is turned on full force. In addition, distention of the jugular veins is more obvious with true jugular distention as found in right heart failure (Figure 3-1). When confusion exists, the jugular vein may be held off near the ramus of the mandible, blood forced distally toward the thoracic inlet, and the vein observed. Emptying the vein in this fashion will eliminate a false jugular pulse, but a true jugular pulse will refill the emptied vein quickly and indicates right heart failure, increased central venous pressure, or right AV valve insufficiency. Some examiners suggest applying light pressure that partially occludes the jugular vein at the thoracic inlet, thereby mildly distending the jugular vein. This is thought to eliminate false (or normal) jugular venous pulsations from a referred carotid arterial impact. In general, the degree of gross distention of the jugular veins in cattle having right heart failure is more impressive than the degree of pulsation (see video clip 1).

Figure 3-1 Obvious distention of the jugular vein in a cow having heart failure secondary to endocarditis.
S2 usually is not as loud as S1 and coincides with aortic and pulmonic valve closure. Current theory suggests that valve closing sounds associated with the generation of S2 result from the sudden halt in valve motion when it closes. Asynchronous closure of the aortic and pulmonic valves results in audible splitting of S2 in many normal cattle, especially during the inspiratory phase of the respiratory cycle.
As in other species, bovine heart murmurs are classified based on intensity and timing. Intensity may be ranked subjectively on a 1 to 6 basis, with 1 of 6 being a faint, barely detectable murmur; 2 of 6 as soft but easily discernable; 3 of 6 as low to moderate intensity; 4 of 6 moderate but lacking a thrill; 5 of 6 loud with palpable thrill; and 6 of 6 so loud that it can be heard with the stethoscope off the chest and evincing a palpable thrill. Classification relative to timing of the cardiac cycle further defines murmurs as systolic, diastolic, or continuous. Further division is provided by terms such as “early systolic” or “holosystolic.” In general, systolic murmurs in cattle reflect AV valve insufficiency or, much less commonly, aortic or pulmonic stenosis, whereas diastolic murmurs reflect aortic or pulmonic valve insufficiency or rarely AV valve abnormalities. Benign systolic murmurs occasionally are heard in excited, tachycardiac calves or cows with anemia, hypoproteinemia, or in those being given rapid intravenous (IV) infusions of balanced fluids. Pathologic systolic murmurs most commonly are found in calves with congenital heart abnormalities such as ventricular septal defect or tetralogy of Fallot and in adult cows with endocarditis. Continuous murmurs are rare but may be encountered in calves having a patent ductus arteriosus or in cows with pericarditis. The point of maximal intensity for each cardiac murmur may add subjective data as to the valve involved in the cardiac abnormality.
In addition to the general signs, specific cardiac signs such as a murmur, arrhythmia, or abnormal intensity of heart sounds usually are present and contribute to the diagnosis. Probably the most difficult set of differential diagnoses involves diseases that result in hypoproteinemia. Hypoproteinemia also causes ventral edema and may cause exercise intolerance and tachycardia. However, hypoproteinemia would not cause jugular and mammary vein distention and pulsation. Therefore venous distention and pulsation coupled with abnormal heart sounds or rhythm are the key signs when diagnosing heart failure in dairy cattle.
Ancillary Procedures
Electrocardiography
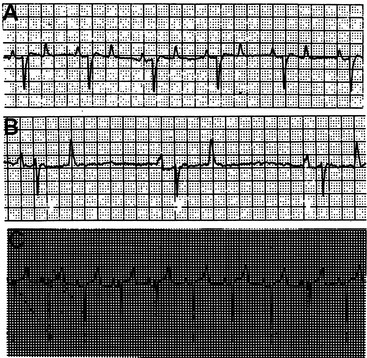
The base-apex lead system is most commonly used in cattle. The base-apex lead system results in an ECG with large wave amplitude and is sufficient for evaluating most arrhythmias. The positive electrode is placed on the skin over the left fifth intercostal space at the level of the elbow; the negative electrode is placed on the skin over the right jugular furrow roughly 30 cm from the thoracic inlet; and the ground electrode is attached to the neck or withers. The resultant ECG recorded through the base-apex lead system has a positive P wave with a single peak, a QRS complex with an initial positive deflection followed by a large negative deflection, and a variable (positive or negative) T wave (Figure 3-3, A to C).
Echocardiography
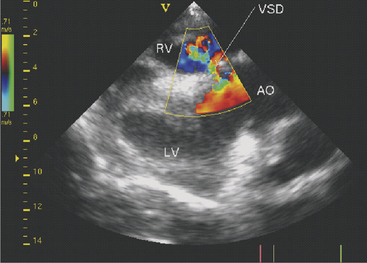
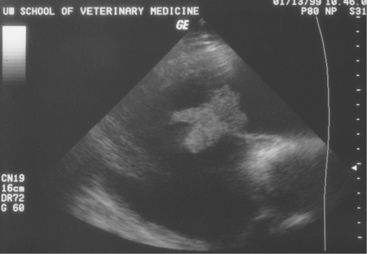
Two-dimensional echocardiography and Doppler echocardiography have greatly enhanced our ability to assess cardiac function and visualize anatomic variations and pathologic lesions in cattle. Valvular, myocardial, pericardial, congenital, and acquired lesions can be visualized in real time, measured, and monitored. Qualitative and quantitative assessment of the impact of congenital anomalies and monitoring treatment response of endocarditis, pericarditis, or other myocardial lesions are possible with the appropriate equipment and people trained to conduct and interpret a systematic cardiac examination. In short, echocardiography is now an essential component of a cardiology workup. Sector scanners utilizing a 3.5-mHz (or lower) transducer are most useful for adult cattle. A 5.0-mHz transducer may be helpful when evaluating neonatal calves suspected of having congenital anomalies or other cardiac conditions. Although some clinicians may lack the equipment or expertise with echocardiography, current graduates are being trained in the technique, and continued competition among manufacturers may allow more veterinarians to own this equipment. In any event, referral for echocardiography is indicated for valuable cattle whenever cardiac disease is apparent but a specific diagnosis is lacking. In most cattle, a systematic examination can be conducted from the right parasternal window to provide a long axis four-chamber view of the heart, a long axis view of the left ventricular outflow tract, and a short axis view of the left ventricle just ventral to the mitral valve and at the papillary muscle level. From the same window, all four heart valves can be visualized, chamber sizes can be measured, myocardial functional measurements can be made, and abnormalities of the pericardium can be seen. Congenital lesions like ventricular septal defect (VSD) (Figure 3-4) and acquired pericarditis (Figure 3-5) are easily visualized by echocardiogram.
SPECIFIC CARDIAC DISEASES IN CALVES
White Muscle Disease
Myocardial damage from vitamin E and selenium deficiency may occur at any site in the heart and may be focal, multifocal, or diffuse (Figure 3-6). Signs may develop at any time from birth to 4 years of age but are more common in calves less than 3 months of age. Specific cardiac signs are variable and include arrhythmias, murmurs, exercise intolerance, cyanosis, dyspnea, congestive heart failure signs, and acute death. Signs may be subtle or dramatic, depending on the magnitude and locations of myocardial damage. Sudden death can occur spontaneously or following exercise or restraint. Other signs of white muscle disease such as stiffness, difficulty in prehension or swallowing, inhalation pneumonia, and myoglobinuria may or may not be present. Dyspnea may be directly related to the cardiac lesions or may be caused by Zenker’s degeneration in the diaphragm or intercostal muscles. Tachycardia (.120 beats/min) and arrhythmias are the most common specific cardiac signs, but murmurs may be present as well.
Treatment should be instituted immediately with vitamin E and selenium injected at the manufacturer’s recommended dosage. Although some commercial preparations include label instructions that include IV use, it is suggested that vitamin E/selenium be given intramuscularly (IM) or subcutaneously to avoid the occasional life-threatening anaphylactic-type reaction seen with these products. The calf should be kept in a small box stall, straw bale enclosure, or hutch, so it can move about but not run freely, lest further muscle damage be precipitated. If pulmonary edema is present, furosemide (0.5 to 1.0 mg/kg) may be given once or twice daily. Concurrent aspiration pneumonia would require intense antibiotic therapy. Vitamin E and selenium injections are repeated at 72-hour intervals for three or four total treatments. Herd selenium status and preventive measures to address the problem should be discussed. Calves that survive for 3 days following diagnosis have a good prognosis.
Hyperkalemia
Hyperkalemia reduces the resting membrane potential, which initially makes cells more excitable, but gradually (with further elevation in potassium and further reduction in resting membrane potential) the cells become less excitable. Atrial myocytes seem more sensitive to these effects than those within the ventricles. Cardiac conduction is affected, and several characteristic ECG findings evolve in a typical sequence that correlates well with increasing K+ values: ECG changes include peaking of the T wave, shortening and widening of the P wave, prolongation of the PR interval, eventual disappearance of the P wave, widening of the QRS complex, and irregular R-R intervals (Figure 3-7). Atrial standstill characterized by bradycardia and absence of P waves may occur and has been documented in association with hyperkalemia in diarrheic calves. Further progression may lead to AV block, escape beats, ventricular fibrillation, asystole, and death.
Congenital Heart Disease
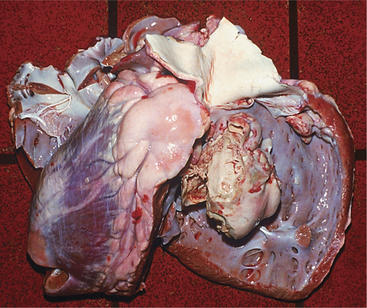
Virtually all types of congenital cardiac anomalies occur in cattle. Most congenital anomalies appear to be sporadic, but inheritance may play a part in some of the most common anomalies. The most common congenital anomalies in cattle appear to be VSDs (Figure 3-8), tetralogy of Fallot, atrial septal defects, and transpositions of great vessels.
Most calves with congenital cardiac defects appear normal at birth but eventually are noticed to have dyspnea, poor growth, or both. Many calves with congenital heart defects are eventually examined by a veterinarian because of persistent or recurrent respiratory signs or generalized ill thrift. The respiratory signs may be real in the form of pulmonary edema associated with heart failure and shunts or be caused by opportunistic bacterial pneumonia secondary to pulmonary edema and compromise of lower airway defense mechanisms. The owners may already have treated the calf one or more times for coughing, dyspnea, and fever, only to have the signs recur. Usually only one calf is affected, thus making enzootic pneumonia an unlikely diagnosis. Regardless of whether pulmonary edema or pneumonia plus pulmonary edema are present, veterinary examination usually detects the cardiac murmur that allows diagnosis. Venous pulsation and distention of the jugular veins may be present, but calves seldom show ventral edema as distinctly as adult cattle with heart failure.
VSDs are the most common defects in dairy calves and are found in all breeds. In Guernseys and Holsteins, VSD may be linked to ocular and tail anomalies. Microphthalmos and tail defects, including absence of tail, wry tail, or short tail, frequently signal VSD (Figure 3-9). Sometimes ocular, tail, and cardiac defects all are present in the same calf, but it is more common to find tail or ocular pathology plus VSD. Depending on the size of the VSD, affected calves have a variable life span. Prognosis for most is hopeless because of eventual respiratory difficulty and stunting. However, calves do, in rare instances, survive to productive adult states. The genetics of these multiple defects (eye, tail, and heart) have not been investigated in Holsteins but have been assumed to be a simple recessive trait in Guernseys.
SPECIFIC CARDIAC DISEASES IN ADULT CATTLE
Neoplasia
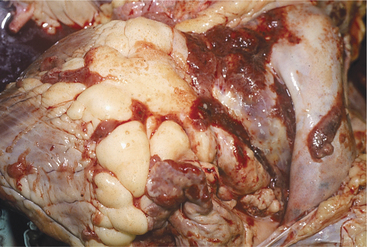
The heart is one of the common target sites of lymphosarcoma in adult dairy cattle. Many cattle with multicentric lymphosarcoma have cardiac infiltration based on gross or histologic pathology, but fewer of these cattle have clinically detectable cardiac disease. When the heart is a major target site, cardiac abnormalities are more obvious. The heart seldom is the only organ affected with lymphosarcoma. Therefore detection of cardiac abnormalities coupled with other suspicious lesions (e.g., enlarged peripheral lymph nodes, exophthalmos, melena, and paresis) simply helps to make a lymphosarcoma diagnosis more definite.
Depending on the anatomic location and magnitude of the tumors, cattle with cardiac lymphosarcoma may have arrhythmias, murmurs, jugular venous distention, jugular venous pulsations, or muffling caused by diffuse cardiac or pericardial involvement. Muffling and splashing sounds are possible if a pericardial transudate or exudate is present. The most common site of tumor involvement is the right atrium, but nodular or infiltrative tumors can be found anywhere in the myocardium or pericardium (Figure 3-10). The color and consistency of the tumors may vary. Mediastinal lymph nodes also are commonly involved. Cattle with signs of heart disease should be thoroughly examined for other lesions consistent with lymphosarcoma. When multiple lesions exist, the diagnosis is easy. However, cattle examined because of vague signs such as hypophagia and decreased milk production that are found to have tachycardia or other cardiac abnormalities can present diagnostic challenges. Although ECG and thoracic radiographs have seldom helped make a definitive diagnosis, ultrasound may be very helpful to image nodular or large masses of lymphosarcoma. Thoracocentesis and pericardiocentesis to obtain fluid for cytologic evaluation are the most helpful ancillary aids when cardiac lymphosarcoma is suspected. A complete blood count (CBC) and assessment of bovine leukemia virus (BLV) antibody status are indicated, but a positive BLV agar gel immunodiffusion (AGID) or radioimmunoassay (RIA) test does not ensure an absolute diagnosis because most positive cattle never develop tumors (see the section on Lymphosarcoma in Chapter 15). Therefore simply assuming a cow with a positive BLV test and heart abnormality detected on physical examination has lymphosarcoma may be an incorrect assumption. A double line-positive BLV-AGID may add further weight to the suspected diagnosis, as would the finding of a persistent lymphocytosis in a set of CBC results. Clinical identification of masses in other locations or cytology from thoracocentesis or pericardiocentesis provides the best means of definitive diagnosis.
Myocardial Disease
Parasitic and Protozoan Infections
Cysticerca bovis may cause myocardial lesions, but these appear rarely in dairy cattle in the northeastern United States. This is the larval form of Taenia saginata, the common human tapeworm. Contamination by human sewage of feedstuffs, pastures, or fields puts cows at risk for this disease.
Sarcocystis sp. is a common cause of myocardial disease in cattle. Although most infestations are asymptomatic, clinical illness characterized by hemolysis, myopathy, myocarditis, weight loss, rattail, and other signs is possible. Sarcocystis sp. requires two hosts, and carnivores or humans usually are the hosts that shed sporocysts in fecal material that subsequently contaminates cattle feed (see Chapter 15). Cattle then become the intermediate host as intermediate stages of the parasite invade endothelial cells and later stages encyst in muscle—including the myocardium. Subsequent ingestion by carnivores of beef containing cysts continues the cycle.
Endocarditis
Etiology
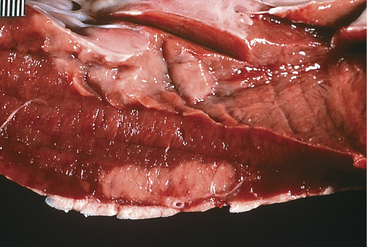
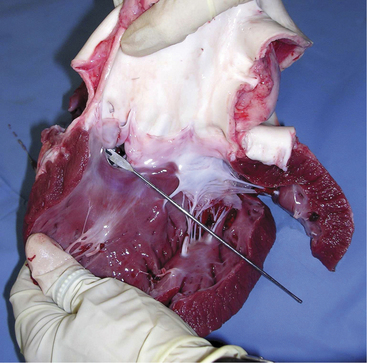
A. pyogenes is the most common organism isolated from the blood and endocardial lesions of cattle affected with endocarditis, but Streptococcus sp., Staphylococcus sp., and gram-negative organisms may also cause the disease. The right AV valve (tricuspid) is the most commonly infected valve with the left AV (mitral) being the second most common (Figure 3-11). Other valves or the endocardium adjacent to valves may also occasionally be the site of infection (Figure 3-12). Owner complaints regarding affected cattle include recurrent fever, weight loss, anorexia, poor production, and sometimes lameness.
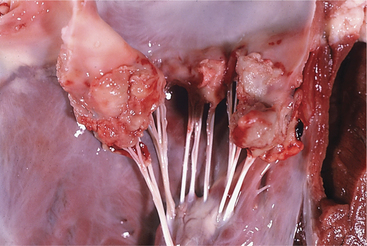
Figure 3-11 Bacterial valvular endocarditis with vegetative lesions in a cow.
(Photo courtesy Dr. John M. King.)
Diagnosis
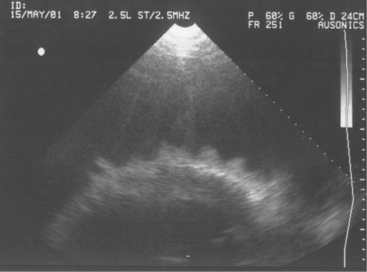
Diagnosis of endocarditis usually is based on the patient’s history and clinical signs. However, a positive blood culture and echocardiography allow definitive diagnosis. Blood cultures, as mentioned previously, may or may not be successful; however, when positive, they allow appropriate selection of antibiotics. Definitive diagnosis based on two-dimensional echocardiography has proven to be one of the most impressive uses of ultrasound since its widespread use in diagnostics began 10 years ago. Veterinarians trained in echocardiography now have a tool to confirm bacterial endocarditis in most patients (Figure 3-13).
Treatment
Treatment continues for a minimum of 3 weeks. Positive signs of improvement include increasing appetite and production, as well as absence of fever. The heart murmur persists and may vary as treatment progresses. Resolution of the heart murmur and tachycardia coupled with echocardiographic evidence of resolution of the endocarditis lesions are excellent prognostic signs. Many cows that survive are, however, left with persistent subtle or obvious heart murmurs caused by valvular damage. This should not be a concern as long as other signs indicate resolution of infection and heart failure is not present. Cattle with venous distention, ventral edema, or other signs of right heart failure have a worse prognosis than cattle diagnosed before signs of heart failure. However, mild to moderate signs of heart failure should not be interpreted to mean a hopeless prognosis because supportive treatment may alleviate these signs while antibiotic therapy treats the primary condition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree