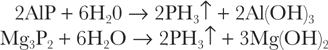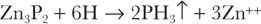Chapter 83 Zinc Phosphide
SOURCES
Zinc phosphide (trizinc diphosphide, Zn3P2) is a metallophosphide that was initially synthesized in 1740. It was first used as a rodenticide in 1911 to control field rodents in Italy and was introduced into the United States around the time of the second World War.1 Zinc phosphide is a dull to lustrous, gray to black crystal that has a faint, pungent, disagreeable phosphorus odor. Commercial zinc phosphide is available as a paste, tracking powder, or grain-based pellets, that generally ranges in concentration from 0.5% to 10%, and is used primarily for the control of rodents, but has also been used as an insecticide.2 When kept in dry, acid-free conditions at up to 40° C (104° F), zinc phosphide is quite stable for at least 2 years, but storage temperatures more than 50° C (122° F) have been shown to cause significant deterioration of the bait.3 The average effective bait life when exposed to wet soil is around 20 days, although the bait may retain some degree of toxicity for several months in the field, even under substantial rain conditions.3,4 In a sufficiently acid environment, zinc phosphide is unstable and hydrolyzes to release phosphine (PH3, hydrogen phosphide) gas, which is spontaneously flammable and is heavier than air.5
Other metallophosphide poisons, including aluminum phosphide (e.g., Fumitoxin and Phostoxin) and magnesium phosphide (e.g., Magtoxin), are used primarily as grain fumigants and are uncommonly encountered by the small animal clinician in North America. Veterinary clinical management for these latter two agents is approached in similar fashion as for zinc phosphide. Zinc phosphide toxicosis in nontarget animals most often results from accidental ingestion of placed baits or malicious baiting acts. Although various animal relay (or secondary) toxicity trials have reported no relay effect, relay toxicosis has been reported from the field in dogs.1,6,7
TOXIC DOSE
Any observed or suspected exposure to zinc phosphide in a pet should be seriously regarded. The minimum toxic dose, minimum lethal dose, or LD50(median lethal dose) of zinc phosphide for the dog or cat has not been reported. Unpublished work indicates that the dose of zinc phosphide producing toxic effects in dogs and cats lies somewhere between 20 and 40 mg/kg body weight (bw).6 In an unpublished trial, a dog dosed with 40 mg/kg bw of zinc phosphide died following acute convulsive activity approximately 7 to 8 hours later.8 Fasted dogs are reportedly more tolerant to zinc phosphide intoxication. One source reported that 100 mg/kg bw of zinc phosphide was lethal in dogs fasted for 24 hours, and another source noted that fasted dogs survived 300 mg/kg bw of zinc phosphide ingestion.6,8 Some pet species that are unable to vomit (rabbits and pocket pets, such as rats, hamsters, and gerbils) and thus forced to retain ingested bait in the stomach are especially susceptible to poisoning.
Various reported animal LD50 values for zinc phosphide include: avian spp. (7.5 to 20 mg/kg bw), jackrabbit (8.25 mg/kg), rat (20 to 40 mg/kg), goat (20 mg/kg), and pig (40 mg/kg).1,6,9 When the only available toxic dose information for a given agent is the LD50, one may wish to apply a factor of one tenth (0.1) to the lowest reported LD50 for the animal species or type in question to assume some degree of a general exposure risk amount or dose, bearing in mind that this rule cannot be relied on as a scientific approach in determining toxicity.10 As an example, by applying the 0.1 rule to the lowest available avian LD50of 7.5 mg/kg bw zinc phosphide, a bird (of no particular species) weighing 1 kg could be considered at risk following ingestion of ∼0.75 mg of zinc phosphide or less than one pellet of a 2% commercial bait product. Zinc phosphide should not be used for rodent control near aviary environments because rodents have been reported to carry bait pieces in their cheek pouches into aviaries and then deposit the bait into avian feeding dishes.11
TOXICOKINETICS
There have been no formal studies of the toxicokinetics of metal phosphides or phosphine in mammals.6 Zinc phosphide is used as a rodenticide by virtue of its ability to release highly toxic phosphine gas in the stomach. To the veterinary clinician managing a patient exposed to this poison, it is important to recall that a sufficiently acid environment is required for zinc phosphide to release phosphine because zinc phosphide is practically insoluble in neutral pH water conditions.2,5,6,12 If zinc phosphide is used as a fumigant, the acid must be available or supplied for significant hydrolysis and phosphine liberation to occur.6 In contrast, aluminum phosphide and magnesium phosphide are readily soluble in water and will hydrolyze in a neutral pH water environment to release phosphine; thus these two metallophosphides are preferred over zinc phosphide in grain storage areas.6,13 Zinc phosphide hydrolysis has been shown to be negligible in tap water, in a variety of surface waters, and in ocean water over periods of up to 11 days.6 Significant hydrolysis of zinc phosphide occurs in vitro at solutions of pH 4 and below, and phosphine generation from zinc phosphide has been reported negligible in canine gastric contents at a pH of 4.3.6,14 Gastric lumen pH of 1 to 3 would be conducive to phosphine release from zinc phosphide.15
Aluminum phosphide and magnesium phosphide readily hydrolyze in water (or acid)13:
Zinc phosphide requires a sufficiently acid environment for hydrolysis13:
The metallophosphides are corrosive and thus act as strong emetics very early following ingestion.2 This initial effect of zinc phosphide can fortunately serve to limit the degree and severity of intoxication in those animal species that are able to vomit.4,8 Rapid onset of persistent vomiting, often hemorrhagic, is commonly noted in dogs.
Once phosphine is released from zinc phosphide, it is readily absorbed from the gastrointestinal tract, possibly by passive diffusion (phosphine is soluble in most organic solvents) into the systemic circulation.13,16,17 Most absorbed phosphine is eventually excreted via the lung as the parent compound, and some phosphine is slowly and incompletely oxidized to relatively less toxic phosphorus oxides and oxyacids (chiefly hypophosphite and phosphite) and excreted in the urine.2,6,13 Following a single oral dose of zinc phosphide, phosphine in human expired air is negligible after 12 hours, suggesting fairly rapid elimination of parent phosphine from the body.6 Zinc phosphide is also absorbed intact from the gastrointestinal tract as evidenced by recovery from stomach wall, blood, liver, and kidneys in humans and in animal studies, where it could be further hydrolyzed to release additional phosphine or oxidized to oxyacid salts.6,13 Absorbed zinc phosphide may remain intact in the body for a longer period of time, possibly beyond 24 hours, and may play a role in delayed hepatic and renal effects that are sometimes noted.6 Dermal absorption of zinc phosphide is negligible.6
MECHANISM OF TOXICITY
The corrosive action of zinc phosphide accounts for the early, acute, and generally hemorrhagic emetic effect on the gastric mucosa.6,18
The systemic toxicity of zinc phosphide is fully accounted for by the toxicity of phosphine, a known and dangerous cytotoxin, which is released from zinc phosphide in the stomach, then rapidly absorbed and distributed throughout the systemic circulation.2,8 Phosphine is a strong reducing compound; readily complexes with metal ions, such as copper; interrupts cellular aerobic respiration; and results in the generation of reactive oxygen species (ROS) with resultant oxidative stress, damage to cell lipids, proteins, and nucleic acids, and cell death.6,19,20 This effect is especially significant in tissues of high oxygen demand (heart, brain, kidney, and liver) or high phosphine concentration (lung).14 Collapse of cell structure and function in individual tissue results in systemic organ failure, and shock.21 Phosphine denatures mammalian hemoglobin and causes methemoglobinemia in laboratory animals.22 Phosphine has also been shown to inhibit acetylcholinesterase in laboratory animals, resulting in cholinergic overdrive.23
CLINICAL SIGNS
Following ingestion of a toxic amount of zinc phosphide bait, initial signs caused by the emetic action of the poison can be noted within 15 to 60 minutes. Clinical signs may delay to 4 hours and rarely to 12 to 18 hours or longer. In severe intoxications, death can occur within 3 to 5 hours.8 Initially the patient is noted to be depressed, anorexic, and nauseated, followed by repeated episodes of vomiting, often with frank or dark blood clots and persistent retching. There may be a garlicky or rotten fish odor (caution: phosphine gas) to the vomitus or patient’s breath. This effect on the gastrointestinal tract can be intensely painful; the patient may vocalize, become irritable, and snap at the handler.8,24,25
As phosphine gas is absorbed systemically, cellular hypoxia develops, which results in cardiopulmonary, circulatory, and neuromuscular compromise. Apprehension, anxiety, discomfort and pacing, weakness and ataxia, labored and gasping respirations, struggling, convulsions, and collapse can occur over a relatively short time span. With progression of the phosphine effect, increased vocalization, frantic and aimless pacing or running, teeth baring or grinding, abdominal straining, and urinary and bowel incontinence may develop. Respirations become rapid, deep, and harsh. Generalized muscle fasciculation and tremor, exaggerated response to external stimuli, involuntary head and neck movements, disorientation, recumbency, thrashing, and convulsive activity may occur.7,8,24,25
On examination the patient is clinically depressed, agitated, apprehensive, and may be delirious. There is hypersalivation and constant retching or vomiting, very often bloody. Lung sounds may be harsh and respiratory effort increased. Tachyarrhythmias and/or bradyarrhythmias may be notable on auscultation. There is general and epigastric pain, and the abdomen is typically tense and splinting on palpation. The patient is typically hyperesthetic, agitated, and irritable. The patient may be brought in recumbent and comatose and exhibiting tonic-clonic or extensor convulsive activity. There may be a slight, offensive, garlicky odor to the patient’s breath (caution: phosphine). In larger exposures, there is generally either notable peripheral hypertension, or hypotension and shock, and moderate to marked hyperthermia from excessive neuromuscular stimulation. There may be physical signs of methemoglobinemia (dark-brown or gun-metal membrane color and chocolate colored blood).7,8,24,25
MINIMUM DATABASE
CONFIRMATORY TESTS
Because phosphine gas dissipates rapidly into ambient air, after collecting a tissue set for histopathology, the entire carcass and any suspect bait and vomitus should be kept frozen and submitted as quickly as possible to a diagnostic laboratory for phosphine analysis. In lieu of the complete carcass, collect and freeze stomach contents and vomitus, suspect bait, liver, and kidney in an airtight container to prevent loss of phosphine gas.14,50 Zinc levels may be elevated in gastric contents, liver, and kidney. Consider a cholinesterase inhibition assay using whole blood (EDTA or heparin) from the live animal or chilled intact eyeball and complete hemisphere of chilled brain postmortem to assess metallophosphide cholinesterase inhibition and to rule out possible organophosphate or carbamate insecticide if the diagnosis is open.
Gas chromatography is a very sensitive method for the determination of phosphine content in samples.13 Dräger Detector Tubes are also used in the veterinary diagnostic setting.14,50 The use of silver nitrate paper has been used in human metallophosphide poisoning cases as a rapid, qualitative bench top screening tool for the presence of phosphine in gastric contents or in the patient’s expired air.17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree