Chapter 14 Adverse Drug Reactions
Adverse drug reactions are an ever-present threat when drugs are used in clinical practice. An adverse drug reaction (ADR) is any noxious or unintended response to a drug that occurs at appropriate doses used for prophylaxis, diagnosis, or therapy. They may vary from minor annoyances to severe, life-threatening events. Drug toxicity includes all toxicity associated with a drug, including that observed in overdose situations (e.g., poisonings with drugs). Side effects generally refers to nondeleterious effects that may occur during therapy, such as polydipsia and/or polyuria in dogs on corticosteroids. Lack of therapeutic efficacy may also be an ADR. However, lack of response may also be caused by an incorrect diagnosis or inappropriate treatment and so is not necessarily an ADR. The frequency with which ADRs occur in the average clinical veterinary practice or in teaching hospitals is not known, but it is generally accepted that ADR is a significant contributor to patient morbidity and mortality.
ASSESSMENT OF RISK
The decision to use a drug is based on a risk-benefit analysis for the individual patient. No drug is without some risk; however, the willingness of the owner and the veterinarian to accept the risk associated with a therapy is dependent on the relative risks and benefits of the drug compared with the risk of no treatment or the risk associated with alternative treatments, such as surgery. A drug should not be used without a specific therapeutic goal, so that efficacy and toxicity can be balanced appropriately.
Although mechanisms are in place for reviewing and recording ADR of licensed products, information for drugs used off-label is less readily available. Many standard veterinary textbooks list adverse reactions that have been reported to drugs without incorporating information on species differences or indeed noting if the adverse reactions have been reported in veterinary species. Further, information on the frequency and severity of ADRs is often lacking. For licensed animal products, the company marketing the product is a good source of information, either through information on the package insert or through direct contact with the company. The Center for Veterinary Medicine, U.S. Food and Drug Administration and the Veterinary Drugs Directorate, Health Canada maintain a record of adverse events that have been reported and use this information to recommend changes in drug labels when appropriate. The FDA’s database is available through its website (http://www.fda.gov/cvm/index/ade/ADER-eport.htm) and is a good source of up-to-date information on potential ADRs that have been reported.
In summary, to make the most use of the information available, to tailor our decisions to the individual patient, and to make rational clinical decisions, an understanding of the basic principles of ADRs is invaluable. Therefore this chapter will first present general principles that can be applied in many clinical situations to guide therapeutic decisions. This will be followed by a brief overview of hepatic and renal ADRs.
CLASSIFICATION OF ADVERSE DRUG REACTIONS
Dose-dependent adverse drug reactions
The occurrence of a dose-dependent toxicity in a patient is not necessarily an absolute contraindication to future use of the drug. If possible, the reason for the occurrence of the ADR should be ascertained. For example, was a dosing error made or was the ADR the result of a drug interaction?
Pharmacological toxicity
Pharmacological toxicity (also referred to as mechanism-based, receptor-mediated, augmented, or Type A adverse reactions) is a form of dose-dependent ADR that arises through exaggerated or undesirable pharmacological effects of a drug (Box 14-1). Pharmacological toxicity is dependent on an interaction of the parent drug or a pharmacologically active metabolite with a specific target or receptor. These effects may be related to the intended therapeutic target, or to additional, inseparable secondary pharmacological actions. In the latter instance, the ADRs are often called “side effects.” For example, a minor side effect would be mydriasis associated with the use of atropine as a preanesthetic agent.
Intrinsic toxicity
Intrinsic toxicity is determined by the chemical properties of the drug, not its pharmacological properties. That is, the toxicity is dependent on the intrinsic chemical properties of the drug—hence the term intrinsic toxicity. The drug or its metabolites do not bind to specific receptors to cause these toxicities, but instead bind nonspecifically to a variety of proteins or nucleic acids, or disrupt membranes or organelle function (Box 14-2). Intrinsic toxicity may have a short time course (e.g., acetaminophen toxicity) or a longer time course (e.g., bone marrow suppression with chemotherapy). It is also referred to as Type A (augmented) or Type C (chronic) adverse reactions, depending on the nature and time course of the reaction.
Box 14-2 Examples of Intrinsic Toxicities
Aminoglycoside nephrotoxicity and ototoxicity
Acetaminophen methemoglobinemia/hemolytic anemia
Sulfonamide-induced hypothyroidism
Clinical pharmacology of dose-dependent adverse drug reactions
Dose-dependent ADRs have the potential to occur in all patients, but they may be avoided in many instances by careful selection of the dose, taking into account the patient characteristics. Patient evaluation becomes very important in deciding whether an adjustment in the recommended standard dose is required. Susceptibility to dose-dependent ADRs can be enhanced through factors which lead to greater drug exposure (i.e., decreased clearance and increased absorption) or that enhance the pharmacological effect (e.g., concurrent medications; presence of epileptic foci in the brain). This hypersusceptibility may also be referred to as patient idiosyncrasy. For example, hypersusceptibility of collie dogs to ivermectin neurotoxicosis is related to an increased penetration of ivermectin into the central nervous system resulting from a genetic variation in P-glycoprotein responsible for pumping ivermectin out of the central nervous system.1,2 Inhibition of metabolism or clearance of a drug can lead to accumulation to toxic levels. Glucocorticoids and nonsteroidal antiinflammatory drugs (NSAIDs) have synergistic effects on the occurrence of gastropathy. In the case of intrinsic toxicities that are dependent on bioactivation to toxic metabolites (a process called bioactivation), factors which alter metabolism of the drug or affect cell defense mechanisms (e.g., deplete cellular glutathione) will also enhance susceptibility.
Treatment in dose-dependent toxicities should involve discontinuation of the drug and, if clinically indicated, removal of the drug from the body through appropriate measures. When appropriate, therapy can be directed at the specific pharmacological target to either treat or prevent the ADR. Targeting to the appropriate pharmacological target is critical. For example, misoprostol is the best and most effective therapy to prevent NSAID-induced gastropathy.3 Once ulcers or erosions have occurred, discontinuation of the NSAID followed by appropriate therapy with sucralfate or an acid inhibitor such as ranitidine or omeprazole would be appropriate. On the other hand, since loss of prostaglandin is not the primary mechanism behind steroid-induced gastric bleeding, misoprostol is not effective in preventing steroid-induced gastropathy.4,5
For intrinsic toxicities, drug withdrawal and supportive care are the most important steps. In certain cases, treatment directed at supporting specific cell defense mechanisms may be appropriate. N-acetylcysteine can function both as an antioxidant to alleviate methemoglobinemia associated with acetaminophen toxicity and as a precursor for glutathione to scavenge reactive metabolites associated with hepatotoxicity.6 Other antioxidants can also be employed to minimize the hematological toxicity associated with acetaminophen.
In summary, dose-dependent ADRs are the most common class of ADRs encountered clinically. They can be minimized by careful and judicious use of the drug, taking into account the individual patient. The clinical manifestation and treatment will be directed by the pharmacological properties of the drug or the mechanism of the chemically based toxicity and the target organ. The previous occurrence of a dose-dependent ADR in an animal is a clear indication for modification of the therapeutic regimen, but does not necessarily contraindicate the use of the causative or a related drug in the patient.
Idiosyncratic adverse drug reactions
Idiosyncratic ADRs are the second major class of ADRs. They are also referred to as host-dependent, dose-independent, Type B (bizarre), Type II, or patient-related ADR. These terms are often used interchangeably (Box 14-3). Unfortunately, because of our lack of understanding of the pathogenesis of many idiosyncratic adverse drug reactions, there remains considerable confusion regarding idiosyncratic reactions. Many clinicians use the term “idiosyncratic” to denote “unknown mechanism.” This, however, is an inappropriate use of the term, particularly as the mechanisms of some idiosyncratic ADRs become elucidated. The defining characteristic of idiosyncratic ADRs is that they occur in patients at serum concentrations within the therapeutic range and will not occur in the majority of patients despite increasing the dose to otherwise toxic levels. That is, a specific interaction must occur between the patient and the drug to result in the adverse reaction. They are not classically dose-dependent and are highly dependent on the characteristics of the individual patient (host-dependent or patient-related). They usually cannot be reliably reproduced in an experimental setting. Thus both experimentally and in the clinical setting, their occurrence is unpredictable. The incidence of idiosyncratic ADRs is usually much lower than dose-dependent ADRs, but in certain populations they may be relatively frequent. Idiosyncratic ADRs are dependent on the chemical properties, not the pharmacological properties, of the drug. They are distinguished from hypersusceptibility to pharmacological or intrinsic toxicities in that they cannot be produced simply by elevating the dose or increasing the exposure in the target population or in experimental animals.
Box 14-3 Examples of Idiosyncratic Adverse Drug Reactions in Veterinary Species
Propylthiouracil/methimazole toxicity in cats
Sulfonamide polyarthritis, thrombocytopenia, hepatotoxicity in dogs
Diazepam hepatotoxicity in cats
Mebendazole hepatotoxicity in dogs
Malignant hyperthermia triggered by halothane in pigs and dogs
Drug hypersensitivity syndrome reactions, drug-induced hemolytic anemia or thrombocytopenia, drug-induced lupus, drug fever, and drug-induced immune-mediated hepatitis are all terms used to describe idiosyncratic reactions that are thought to have an immunological basis. The clinical manifestations of “idiosyncratic hypersensitivity syndrome reactions” include such pathological states as fever, lymphadenopathy, dermatopathies, hepatitis, nephritis, leucopenia, agranulocytosis, eosinophilia, thrombocytopenia, and aplastic anemia. This type of idiosyncratic reaction is relatively rare (frequency estimated to be <1/1000) and has a delayed onset, with clinical signs generally manifesting 7 to 14 days or longer after the start of therapy.7 They are distinct from the typical drug allergy characterized by anaphylaxis and/or urticaria occurring immediately after drug administration, which is an IgE-mediated immediate hypersensitivity reaction directed against the drug.
Idiosyncratic reactions are important in veterinary medicine from a patient treatment standpoint, but they also have an influence on veterinary practice from another perspective. Fear of idiosyncratic toxicity in humans may be the reason for the banning of products for use in food animals (e.g., chloramphenicol causes aplastic anemia in rare individuals) or may lead to the withdrawal of a drug from the market. Some practitioners are reluctant to prescribe drugs that have been associated with idiosyncratic ADR in humans for fear of precipitating an event in the owner. In general, owners should be warned about the potential for drugs employed in veterinary practice to cause idiosyncratic reactions in humans (Box 14-4) and be instructed to wash their hands immediately after administering the drug to their animals. It is wise to inquire if the client or any immediate family members have drug allergies before dispensing a drug so that they can take appropriate precautions, such as wearing gloves and washing hands.
Pathogenesis of idiosyncratic adverse drug reactions
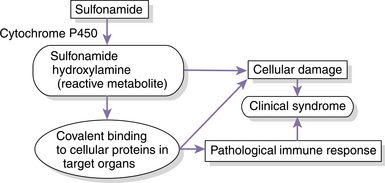
The pathogenesis of idiosyncratic ADRs is complex and is dependent on the reaction under consideration. For example, malignant hyperthermia is related primarily to mutations in the ryanodine receptor in the muscle sarcoplasmic reticulum8 so that muscle calcium homeostasis cannot be maintained in the face of challenge with certain muscle relaxants, caffeine, and halothane. It is an idiosyncratic reaction because it requires a specific patient genotype and, although a mutated receptor is responsible for susceptibility, interaction of halothane with a specific receptor is not required to trigger the clinical event. The most common types of idiosyncratic reaction, however, involve cellular damage, leading to organ specific damage, such as nephropathies, hepatopathies, blood dyscrasias, and dermatopathies. These reactions are commonly dependent on bioactivation to a reactive intermediate that can either directly cause cellular damage or trigger a pathological immune response.
Clinical signs consistent with an immunological pathogenesis for many idiosyncratic reactions include a delayed onset, typically 7 to 14 days after the start of therapy, fever, skin rash, and occasionally eosinophilia. The clinical signs are highly variable, depending on the patient and other clinical factors. Patients may display a clearly systemic disease with multiple organs affected, or may have a single abnormality, such as thrombocytopenia, neutropenia, skin rash, or hepatitis. A previous exposure to the drug may have occurred, but is not necessary. If an animal has tolerated a drug for more than 6 to 8 weeks, the likelihood of experiencing an idiosyncratic reaction drops. Despite the variable clinical presentation, it appears that common pathogenic events underlie the clinical disease.
The immunological responses that have been identified in cases of idiosyncratic reactions in humans and animals have been directed against either drug-modified proteins or autoantigens. Drugs are themselves generally too small to trigger an immunological response; however, if they are metabolized to reactive metabolites, they may form drug-protein conjugates (Fig. 14-1) that are capable of triggering an immunological response.3,9 The immune response may be directed against the drug-protein conjugate or against the protein itself (autoantigen) that was altered by the drug. The factors that determine which animals will experience an idiosyncratic reaction remain obscure, although genetic and environmental differences in metabolic capacity and immunological responsiveness appear to play roles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree