J. Richard Newton, Debra Elton, Ann Cullinane
World Status of Equine Influenza
Equine influenza (EI) continues to be an important disease across most of the globe, primarily because of its ability to cause extensive disruption to equestrian breeding, training, and competition schedules. As a result of this economically important threat, vaccination against EI has become widespread and mandatory in some equestrian disciplines. Vaccination became mandatory for racing Thoroughbreds in the United Kingdom, Ireland, and France in 1981, following the epizootics of 1979; since mandatory vaccination was introduced, major outbreaks have been much less frequent, and no racing days have been lost to the disease. Importantly, however, increasing volumes of international horse air transport and potential transmission from subclinically infected vaccinated horses provide opportunities for very rapid spread of EI over long distances, even between continents, if high levels of biosecurity are not strictly observed. The incursion of the H3N8 EI virus into Australia in 2007 serves as a reminder of the impact that EI can have when it enters a large population of unvaccinated horses. It also highlights the importance of maintaining global surveillance, optimal vaccine composition, robust preexport methods of accurately ensuring freedom from infection, and the highest standards of biosecurity for containing this highly infectious disease.
The Disease and Its Cause
Equine influenza is a highly contagious viral disease that typically manifests in fully susceptible animals (i.e., those with no existing immunity acquired through maternally derived antibody, vaccination, or prior infection) with clinical signs of a frequent, dry, harsh, hacking cough; serous and subsequently mucopurulent nasal discharge; and fever. These signs are associated with viral damage to the respiratory ciliated epithelium, temporary loss of normal mucociliary clearance mechanisms, and increased susceptibility to bacterial infections and allergens. Depression, inappetence, myalgia, edema, and enlarged submandibular lymph nodes may also be observed in more severe cases. Nonspecific changes on hematologic profile may include anemia, leukopenia, and lymphopenia, but these are not pathognomonic for EI. The clinical signs of EI usually resolve over several weeks, and the infection is rarely fatal, although complications associated with secondary infections, pneumonia, or pleuritis may lead to death in some higher-risk individuals. The severity of clinical signs may be related to the virulence of the virus strain. It has been suggested that in rare instances, EI may induce encephalitis in horses, but a definitive link between neurologic abnormalities and infection with EI has not been established.
In populations of susceptible animals, presumptive diagnosis of EI may be accurately made on the basis of very rapid spread of typical clinical signs, in particular the characteristic frequent, dry cough. Clinical signs are usually less severe in vaccinated or partially immune populations of animals and can be mistaken for milder respiratory infections. However, although disease spread can be slower and less extensive than among fully susceptible populations, there is usually rapid spread of the milder signs among the group, particularly nasal discharge, which is typically not a feature of other equine respiratory infections. Subclinical infections with EI virus may occur in well-vaccinated competition horses, although they may be associated with periods of poor athletic performance among this group.
Equine influenza is caused by an orthomyxovirus of genus influenza A, which is divided into serologic subtypes based on the hemagglutinin (HA) and neuraminidase (NA) surface glycoproteins. There are many combinations of the currently known 17 HA and 9 NA subtypes found in wild aquatic birds, but only two subtypes are known to have become established in horses, H3N8 and H7N7. Those currently circulating in horses all belong to the H3N8 subtype and can be traced back phylogenetically to 1963, when it was first isolated from racehorses in Miami. Equine influenza viruses are, like other influenza viruses, named according to their type, host species, subtype, and place and year of isolation (e.g., A/equine 2/Miami 1963 [H3N8]).
Historically, an H7N7 subtype predated the appearance of H3N8 in horses, and although the two subtypes cocirculated for several decades, equine H7N7 viruses have not been isolated for many years and are now considered to be extinct from equids. Since 1979, all outbreaks of EI in horses giving rise to antigenically characterized isolates globally have been attributed to H3N8 viruses, although highly pathogenic avian influenza (HPAI) H5N1 was isolated from clinically affected donkeys in Egypt in 2009. That outbreak, associated with cross-species transmission of HPAI H5N1 from infected poultry, along with a large outbreak of more fatal avian H3N8 in horses in China in 1989, highlights the potential threat posed by cross-species transmission of influenza A viruses from birds to equids.
Global Disease Dynamics
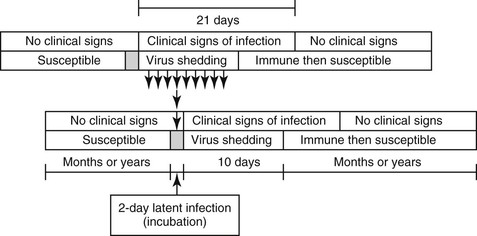
A nonaffected (“healthy” for EI) but susceptible animal encounters infectious EI virus and undergoes relatively short latent (infection to becoming infectious) and incubation (infection to first signs) periods before showing clinical signs and becoming infectious to others (Figure 39-1). The infection is immunogenic and is cleared after 7 to 10 days in most individuals. However, clinical signs, particularly coughing, may take longer to resolve because the ciliated respiratory epithelium must be reestablished. Horses are solidly immune to reinfection for many months after clinical signs have resolved. A persistent subclinical carrier state for EI is not recognized in horses, and persistence of infection within the equine population requires chains of transmission among individual horses. Persistence of infection (endemicity) requires each infectious individual on average to infect at least 1 other animal. It has been calculated that, in epidemic situations such as those seen in the United States in 1963, each infectious horse could lead to as many as 10 newly infected horses. In nonvaccinated susceptible horses, the short incubation period and persistent cough lead to release of large quantities of virus into the environment and contribute to the rapid spread of infection. Horses stabled under intensive conditions are at increasing risk from a buildup of infective virus in the common airspaces. Dispersal of animals from horse shows, sales, race meetings, and other events where EI has spread may lead to widespread dissemination of virus into the wider equine population.
Outbreaks of EI have been reported in most horse populations globally, except for a few island nations. Some, such as New Zealand, have managed to prevent incursion by taking extreme care with horse imports, whereas others, such as Iceland, do not permit horse imports at all. The disease is considered endemic in Europe and North America and has important economic significance to the equine industry worldwide. Imported subclinically infected vaccinated horses, coupled with inadequate quarantine procedures, gave rise to major outbreaks in susceptible populations in South Africa (1986 and 2003), India (1987), Hong Kong (1992), and Australia (2007).
Equine influenza is highly infectious and is spread by the respiratory route, primarily through direct contact between infectious and susceptible horses, although airborne spread over longer distances is possible under favorable conditions. Indirect transmission by personnel and fomites may also contribute to virus spread, as was probably the case in Australia in 2007 when influenza escaped from a postarrival quarantine station even when no horses were released. In South Africa in 1986 and 2003, contaminated vehicles were implicated in the spread of the virus. In the 2007 outbreak in Australia, initial spread of the infection in the susceptible Australian horse population was linked to mixing at and dispersal of horses from a 1-day event held in New South Wales in mid-August. By December 2007, when the last case was diagnosed, more than 76,000 horses on more than 10,000 properties were reported to have been infected.
The Global Picture
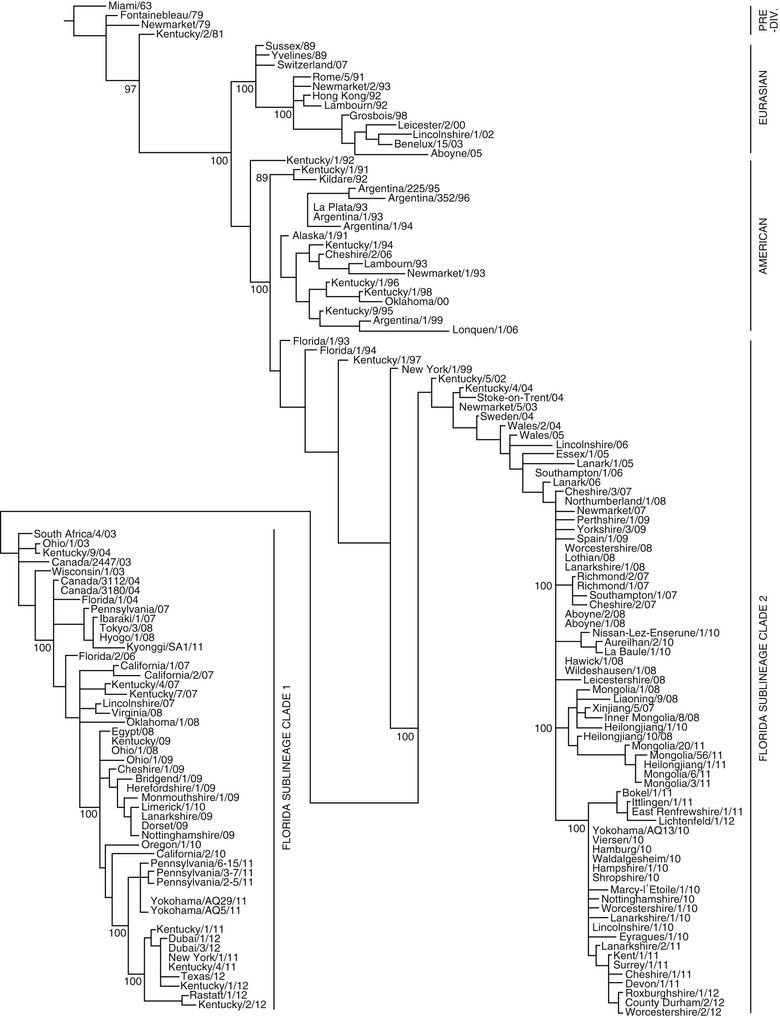
It is recognized that EI viruses, like other influenza viruses, undergo antigenic drift secondary to gradual accumulation of mutations in the genes encoding HA and NA. These mutations eventually lead to significant antigenic changes in the virus that can lead to breakdown of host immunity. Use of HA (or NA, less commonly) gene sequence data from the earliest viruses as a common reference makes it possible to create a family or phylogenetic tree of H3N8 EI viruses, which highlights the evolution of the virus over time (Figure 39-2).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree