Chapter 2 Wistar and Sprague–Dawley rats
Introduction
Almost all laboratory and domestic pet rats belong to a single species, the Norway rat (Rattus norvegicus). The rat is the most commonly used rodent species for toxicology studies. A number of rat species are used in research, contract research organizations and in the pharmaceutical industry including the Wistar, Sprague Dawley and Fischer 344 rat. Different strains of rat differ in their sensitivity to various compounds and in their incidence of spontaneous tumors. Differences in age and sex of rats should also be taken into consideration when designing toxicological and experimental studies (Johnson & Gad 2007). Background lesions in rats are made up of degenerative, inflammatory, proliferative and congenital changes. Although aging rats produce many interesting lesions, there are also a number of lesions that occur spontaneously in young animals.
The pathology of the aged rat becomes important in the latter stages of carcinogenicity studies (Chandra & Frith 1992). The nonneoplastic lesions and proliferations noted in aging rats are problematic as they can be confused with preneoplastic and neoplastic disease (Johnson 2007). This chapter will give an overview of background lesions encountered in both young and older Wistar and Sprague-Dawley rats.
Cardiovascular system
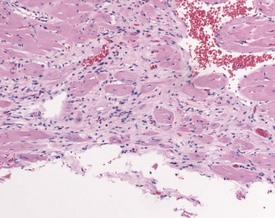
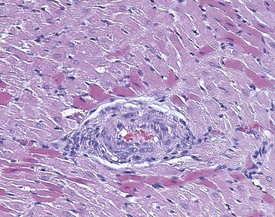
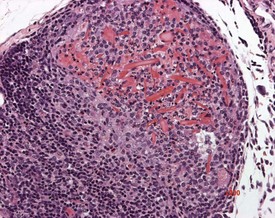
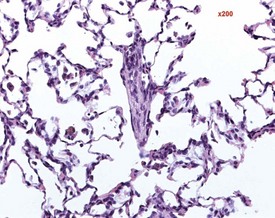
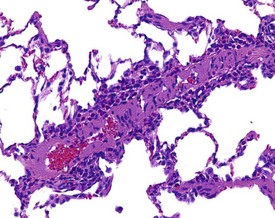
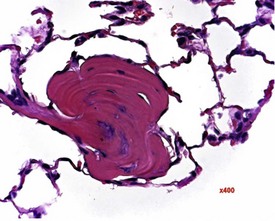
Cardiomyopathy (Fig. 2.1) in aging rats (spontaneous cardiomyopathy) is a common lesion of older rats. It is found most frequently in the subintimal myocardium of the left ventricular wall and is more common in male rats (MacKenzie & Alison 1990). The etiology of the disease is unknown, but severity and age of onset are influenced by diet, environment and stress (MacKenzie & Alison 1990). The lesion is characterized initially by necrotic, eosinophilic cardiomyocytes surrounded by fibroblasts and inflammatory cells (Fig. 2.2), predominantly lymphocytes. Later, fibrosis is prominent in aging rats and is generally present in the left ventricle, intraventricular septum and papillary muscle. Cartilagenous and osseous metaplasia of the chorda tendinae may occur at the end stage of the disease (Ruben 2000). Atrial (left) and ventricular thrombosis are occasionally associated with cardiomyopathy in aging rats.
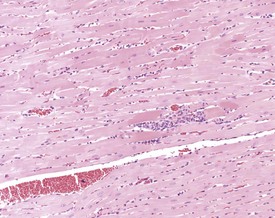
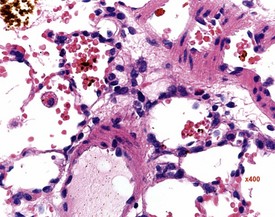
Mesenchymal proliferation in the subendocardium (Fig. 2.3) is also known as endocardial hyperplasia, endocardial disease, endomyocardial disease or subendocardial fibrosis. Although endocardial schwannomas can be found in the same location, it is not known whether the mesenchymal proliferation in subendocardium always progresses to neoplasia (Johnson & Gad 2007). The cells are distinct from the bordering myocardial cells, but may extend into muscle bundles.
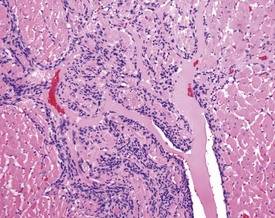
Cartilaginous foci at the base of the aorta are common in both young and old Sprague–Dawley rats (Fig. 2.4) (Johnson & Gad 2007). In older rats the cartilaginous foci may mineralize (MacKenzie & Alison 1990). Adipose tissue infiltration in the heart is characterized by the presence of subepicardial adipose cells and is often observed in older, obese animals (Ruben 2000). Lipofuscinosis results in golden brown pigment granules at the poles of the nuclei of myocytes (Ruben 2000).
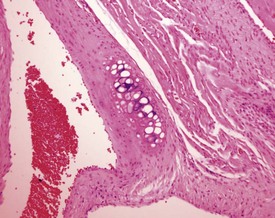
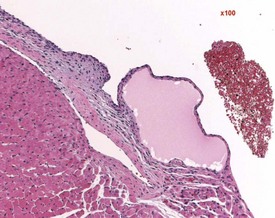
Endocardial myxomatous change occurs in older rats and is recognized by focal or diffuse thickening in the subendocardium due to the presence of myxomatous tissue (Ruben 2000). Hypertrophy and hyperplasia of the endocardium may be seen in conjunction with the myxomatous valvular changes. In addition, the lesion is characterized by focal areas of hemorrhage, hemosiderin pigmentation, hyalinization, thrombi formation, inflammatory and mast cells and polypoid projections (Ruben, 2000). Heart valve cysts may occasionally be observed in the heart valves of rats (Fig. 2.5).
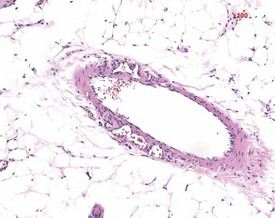
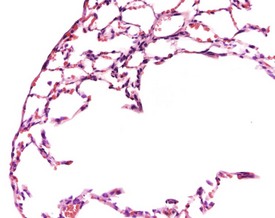
Vascular lesions in the blood vessels of Sprague-Dawley and Wistar rats include medial degeneration and medial hypertrophy. Medial degeneration is common in the coronary arteries of rats and the affected vessels display enlarged, irregularly arranged and sparse nuclei present in a PAS-positive matrix (Ruben 2000). Medial hypertrophy of the blood vessels is rare and tends to occur in pulmonary arteries. This lesion consists of thickening of the tunica media due to hypertrophy of the smooth muscle cells (Ruben 2000) (Fig. 2.6).
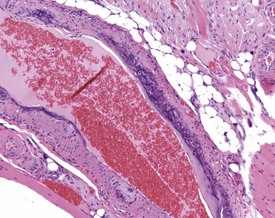
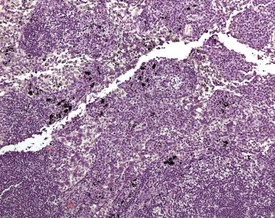
Arteritis (polyarteritis, periarteritis, panarteritis nodosa) (Fig. 2.29) is found occasionally in Wistar rats, but is more common in Fischer rats (MacKenzie & Alison 1990). The occurrence of the lesion is higher in males and increases with age. Pancreatic, mesenteric and spermatic arteries are more commonly affected. The lesion consists of fibrinoid degeneration of the media with inflammatory cell infiltration progressing to fibrosis, vascular dilatation, occlusion and aneurysm (Mackenzie & Alison 1990). The pathogenesis is unknown but may be related to immune complex disease. The vaso vasorum of the mesenteric arteries of the rat can occasionally be confused with a treatment-related finding (Fig. 2.7a). Age-related vascular medial cartilage and mineralization may be encountered in the blood vessels of aging rats (Fig. 2.7b).
Arterial thrombosis (not caused by arteritis) occurs occasionally in the lung, kidney, liver and adrenal (Ruben 2000). These thrombi may be emboli originating from atrial thromboses in older rats. Atherosclerosis is a rare background lesion consisting of focal areas of foam cells within the tunica intima or tunica media (Ruben 2000). Intimal and/or medial sclerosis with mineralization of the elastic fibres occurs in the aorta (Ruben 2000). Vascular mineralization in arteries and veins in various organs such as the lung, is occasionally observed as a background lesion in rats.
Hemolymphoreticular system
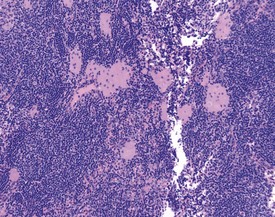
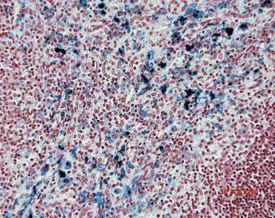
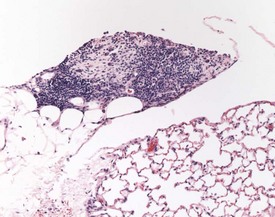
Pigmented macrophages in the sinuses (Fig. 2.8) (particularly the medullary or subscapular sinuses) of the lymph nodes are observed commonly in rats. Generally this pigment is ceroid or lipofuscin material (Stefanski et al 1990). Special stains (such as Schmorl’s for lipofuscin) should be used to distinguish ceroid pigment from iron (Perl’s-positive) or melanin (Masson Fontana-positive).
Cystic degeneration (Fig. 2.9) of various different lymph nodes (also known as sinus dilatation, lymphangiectasia, cystic ectasia, lymphatic sinus ectasia, lymphangiectasis, lymphatic cysts) is observed as a background lesion in rat lymph nodes. Generally the subcapsular and medullary sinuses are dilated. The sinuses are dilated with lymph and occasional lymphocytes or macrophages and erythrocytes or proteinaceous fluid can be observed within the lumen (Stefanski et al 1990). Cystic dilatation of the lymph nodes may be so prominent that it is noted at necropsy. The mesenteric, mediastinal and paralumbar lymph nodes are often affected (Frith et al 2000b). Vascular ectasia (also known as angiectasia, vascular sinus dilatation, peliosis, telangiectasis) is the dilatation of vascular channels within the medullary sinuses (Frith et al 2000b). Thrombosis is occasionally observed in the blood vessels in the lymph node (Frith et al 2000b).
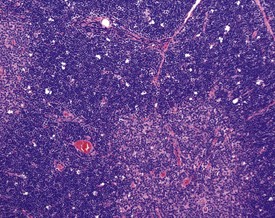
Lymph node atrophy (also known as senescent atrophy) is a common lesion of aging rats. The atrophy is generally characterized by a reduction in germinal centres and follicles and depletion of lymphocytes in the paracortex can also be seen (Stefanski et al 1990). Senescent atrophy is often nearly ubiquitous in carcinogenicity studies. Moderate amounts of intracellular pigment (generally lipofuscin) are often noted in the medullary area.
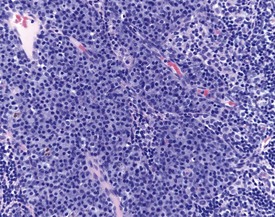
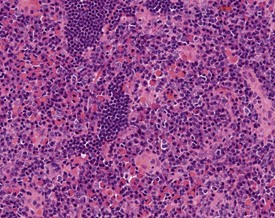
Mast cells in lymph nodes (Fig. 2.10), particularly the mandibular lymph node, are observed frequently in the lymph nodes of both young and old rats. Plasmacytosis of mandibular lymph node (Fig. 2.11) is found predominantly in the medullary cords (Stefanski et al 1990). Plasmacytosis of lymph nodes may be so severe as to be noted at necropsy as enlargement. The etiology and significance of the lesion are unknown, but the lesion is particularly common in mandibular lymph nodes, particularly in conjunction with oral cavity lesions. Plasmacytosis may be prominent in animals with T cell atrophy (Frith et al 2000b). In addition, accumulations of Russell bodies may also be observed in a lymph node with plasmacytosis (Fig. 2.12).
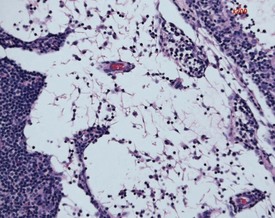
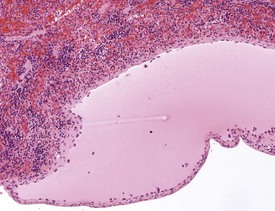
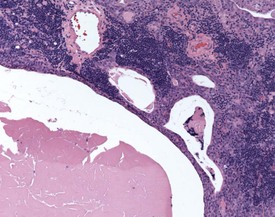
Mineralization of Payer’s patches or other lymph nodes is occasionally observed and is likely to be secondary to tissue damage (Frith et al 2000b) (Fig. 2.13). Small capsular cysts may occur on the surface of the rat spleen (Fig. 2.14). The cysts are lined by endothelial cells and contain a pink proteinaceous fluid (Stefanski et al 1990).
Sinus erythrocytosis is characterized by the presence of erythrocytes within the lymph node sinuses (Stefanski et al 1990). This lesion is common and generally occurs due to the presence of hemorrhage in the organs draining into the lymph nodes. Erythrophagocytosis by macrophages may also be visible within the sinuses containing red blood cells.
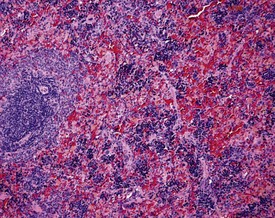
Increased extramedullary hematopoiesis is a common lesion in the rat spleen (Fig. 2.15). The rat spleen normally has a minimal level of extramedullary hematopoiesis; however, it is very difficult to distinguish between normal and excessive levels of extramedullary hematopoiesis. Congestion is a common background lesion of the spleen and may be linked to the method of euthanasia (Frith et al 2000b).
Increased hemosiderin (Fig. 2.16) is observed as a background lesion in rats. The rat spleen, particularly in female animals, normally has minimal to slight amounts of hemosiderin. In common with extramedullary hematopoiesis, it is often difficult to distinguish between normal and excessive amounts of hemosiderin. Lipofuscin pigment may also be present in the red pulp of older rats (Frith et al 2000b). In addition, tattoo pigment which has drained to the peripheral lymph nodes may occasionally be observed in rat lymph nodes (Fig. 2.17).
Accessory spleen is an uncommon background finding in the rat abdomen. Generally it presents with nodules of splenic tissue present within the mesenteric adipose tissue. The cause is either congenital or traumatic injury (Stefanski et al 1990). Mineralization may occasionally be seen in the walls of the blood vessels of the spleen as well as in the capsule of aging rats (Frith et al 2000b). Parenchymal fibrosis of the red pulp of the spleen can occasionally be observed in ageing rats.
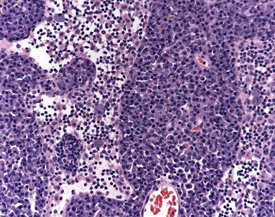
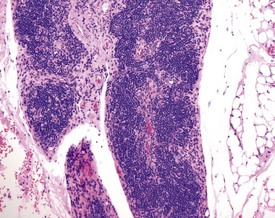
Ectopic thymus is a common finding adjacent to the thyroid. Ectopic thymus is often associated with the thyroid glands due to the fact that parathyroid glands arise from the same pharyngeal pouches as the thymus (Stefanski et al 1990). Thymic cysts (Fig. 2.18) or epithelial remnants are observed as a background change in the rat thymus. These may be made up of epithelial tubules or nests in the medullary region of the thymus in older rats. Occasionally these remnants can give rise to tumors. Some of the cysts are remnants of the thymopharyngeal duct, others are dilatations of thymic tubular structures (Stefanski et al 1990). Thymic cysts are often lined by squamous cells or ciliated epithelium.
Ectopic parathyroid tissue can occasionally be encountered adjacent to the thymus (Frith et al 2000b). Multifocal hemorrhage (and occasionally eosinophilic crystals) is very common in the thymus of rats, particularly as an agonal lesion due to carbon dioxide euthanasia (Frith et al 2000b) (Fig. 2.19). Thymic atrophy (Fig. 2.20) (also known as involution) is a common background finding in aged rats. Although the thymus continues to increase in size initially, it begins to regress and at the end of a carcinogenicity study of two year duration the thymus will be severely depleted (Stefanski et al 1990). As the atrophy progresses, the epithelial components of the thymus become more prominent and often form tubular structures (Stefanski et al 1990).
Thymic cortical apoptosis is a common background finding (Fig. 2.21). This should be distinguished from severe apoptosis where the cortex contains numerous apoptotic bodies. Severe apoptosis is often observed when animals are placed under severe stress or treated with immunosuppressive drugs (Stefanski et al 1990).
Bone marrow atrophy is observed in aging rats and the lesion is characterized by a decrease in hemopoietic cells, an increase in adipocytes and an increase reticular stroma (Frith et al 2000b). Hemosiderin pigment is occasionally observed in the bone marrow and may be associated with previous hemorrhage (Frith et al 2000b).
Germinal centres and lymphoid follicles can occasionally be present in the bone marrow of normal rats (Frith et al 2000b).
Respiratory system
Eosinophilic inclusions (also known as globules and droplets) are observed commonly in aging rats in the nasal epithelium (Renne et al 2003). The inclusions occur in the olfactory, respiratory epithelium and mucous glands. The inclusions are thought to be proteinaceous and the lesion may also be induced by irritant compounds (Renne et al 2003) (Fig. 2.22). Eosinophilic inclusions may occur in association with the loss of sensory cells (Renne et al 2009). These inclusions are negative for periodic acid–Schiff (PAS), Alcian Blue, Von Kossa, mucicarmine, phosphotungstic acid hematoxylin (PTAH), Masson’s trichrome, Congo Red, and toluidine blue stains (Monticello et al 1990). Ultrastructurally, the inclusions appear to be amorphorous, flocculent material in membrane-bound vacuoles (Renne et al 2009).
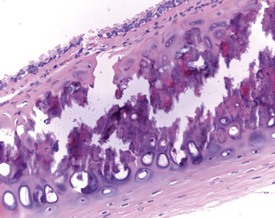
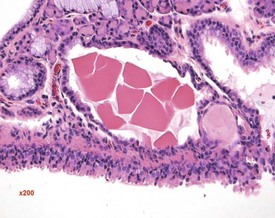
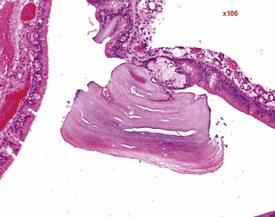
Foci of mineralized concretions or corpora amylacea (Fig. 2.23) are observed commonly in nasal epithelia lining the nasal turbinates (Monticello et al 1990). Amyloid and amyloid-like materials have been observed in various tissues of aged mice of several strains, including the nasal cavity (Renne et al 2009). Osteopetrosis of the nasal turbinates may present as a spontaneous lesion (Renne et al 2003). Globule leukocytes are present in the respiratory epithelium of the larynx and trachea in rats (Renne et al 2003, 2009). Mineralized hair, food particles or debris are often present in the ventral pouch of the rat larynx, particularly in older rats and squamous metaplasia may be recorded in the respiratory epithelium overlaying the ventral gland as a spontaneous lesion in male Wistar rats on inhalation studies (Renne et al 2003). Fischer 344 rats, especially the female sex, exposed to chronic daily irritation by gavage, are predisposed to high mortality rates on chronic studies due to the high incidence of cartilage degeneration, which presumably leads to a dysfunction of the larynx (Germann et al 1998).
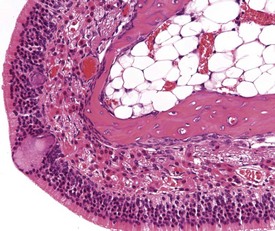
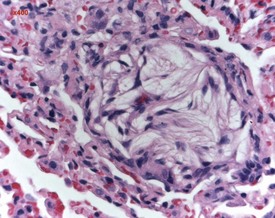
FIGURE 2.23 Mineralization (corpora amylacea) of the olfactory epithelium of the rat nasal turbinate. ×200.
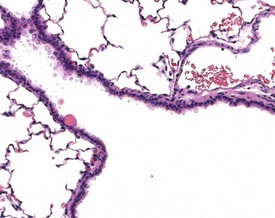
A common background lesion in the rat lung is mineralization of pulmonary arteries (Fig. 2.24). The lesion consists of focal or multifocal mineralization situated beneath the intima or within the muscular walls of the arteries. The lesion is not associated with metastatic calcification.
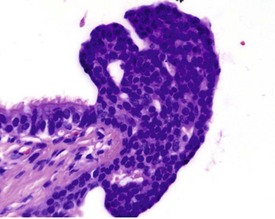
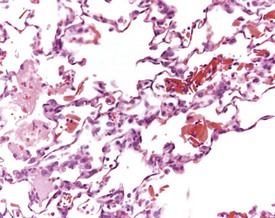
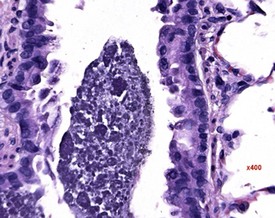
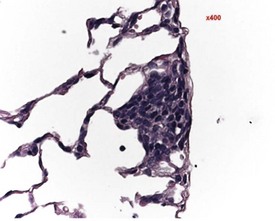
Small areas of alveolar hemorrhage (Fig. 2.25) are often present in the rat lung and represent an agonal event, particularly in rats killed with carbon dioxide or those that die spontaneously (Renne et al 2003). Occasionally, small foci of neuroendocrine cells in the lung (Fig. 2.26) may be visible in aging rats. Pulmonary neuroendocrine cells are found as clusters called neuroepithelial bodies or as single cells scattered in the respiratory epithelium (Haworth et al 2007). Pulmonary neuroendocrine cells are defined as foci of neuroendocrine cells with greater than 40 nuclei (Haworth et al 2007). The cause of the hyperplasia is not known and the lesion is not thought to progress to neoplasia (Haworth et al 2007). Morphologically the lesion resembles chemoreceptors such as carotid bodies (Haworth et al 2007).
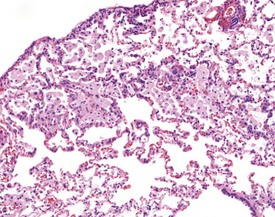
Multifocal aggregates of alveolar macrophages (Fig. 2.27) (also known as alveolar histiocytosis) within alveoli and terminal airways are observed in both young and old rats. The alveolar macrophages often contain abundant, foamy cytoplasm (Boorman & Eustis 1990) and the lesion may be visible at necropsy as white areas on the lung surface (Johnson & Gad 2007). The aggregates are often subpleural or located in the more peripheral regions of the lung (Boorman & Eustis 1990). Cholesterol clefts (Fig. 2.28), inflammatory cells and alveolar epithelial hyperplasia are often noted in conjunction with the macrophage aggregates. The aggregates may represent resolved inflammatory foci and may represent a focal deficit in the pulmonary clearance mechanisms. Some macrophages contain brown pigment which may be hemosiderin (Renne et al 2003) or lipofuscin (Fig. 2.29) or carbon from the atmosphere.
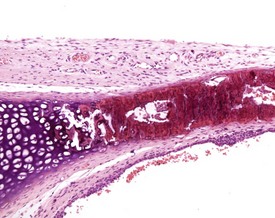
Occasionally pleural tags (Fig. 2.30) are observed in rat lungs. These consist of pleural extensions which often contain mononuclear cells such as lymphocytes. Mineralization of tracheal cartilage (Fig. 2.31) is a common aging change in rats and is observed in most two-year-old rats on carcinogenicity studies. Large eosinophilic cytoplasmic inclusions are occasionally seen in untreated rat (Kambara et al 2009). Clara cells (Fig. 2.32) are generally found in the bronchiolar epithelium. The presence of this deeply eosinophilic solitary cell is often confusing. Perivascular eosinophilic infiltration (Fig. 2.33) is observed commonly in the lungs of young and old rats. The etiology of this finding is unknown.
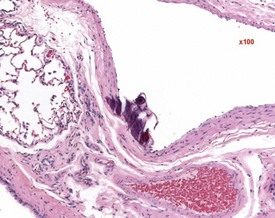
Hair shaft emboli or skin fragment emboli are seen in the lungs of animals on intravenous injection studies. These foreign bodies generally induce a granulomatous reaction consisting of multinucleated giant cells and other inflammatory cells (Renne et al 2003) (Fig. 2.34). Eosinophilic crystals are occasionally observed free within the alveoli (Renne et al 2003) of rat lungs. The crystals may be associated with areas of alveolar hemorrhage (Fig. 2.35). Hyperinflation (Renne et al 2003) or pulmonary acinar ectasia consists of areas of alveolar dilatation and may be observed in the subpleural region in aged rats (Renne et al 2003) (Fig. 2.36a). Isolated cystic spaces in lung parenchyma are rarely seen in rats and are of uncertain origin. These cysts are lined only by fibrous tissue, with no evidence of inflammation (Renne et al 2009). Apparent dilatation of alveoli can also occur due to excessive instillation of fixative into the lung at necropsy (Renne et al 2003).
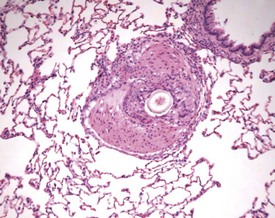
FIGURE 2.34 A hair embolus within an artery in the rat lung, surrounded by inflammatory cells. ×100.
Dilatation of submucosal glands of the larynx or trachea may also occur as a background lesion in rats (Renne et al 2009) (Fig. 2.36b). Foci of osseous metaplasia are commonly observed in the lungs of both young and old rats (Renne et al 2009) (Fig. 2.37). In addition, ossification of the cartilage in the larynx is also occasionally noted in ageing rats (Fig. 2.38). Furthermore, mineralization of mucus is rarely noted within bronchioles of the lungs and within the lumena of the nasal turbinates (Figs 2.39, 2.40). Foci of minimal, generally mononuclear cell inflammation may be noted below the pleura in the rat lung (Fig. 2.41).
Researchers have noticed inflammatory lesions in the lungs of rats used in research in recent years. The lesions consist of perivascular lymphocytes, inflammatory cells and alveolar macrophages in the lung alveoli and the presence of epithelial hyperplasia. These lesions may mask or confuse research findings, particularly in inhalation studies. Initially a virus was suggested as the cause (rat respiratory virus) (Albers et al 2009), but recently researchers have demonstrated a correlation between the presence of inflammatory lung lesions and Pneumocystis carinii DNA detected by PCR (Livingston et al 2011).
Gastrointestinal system
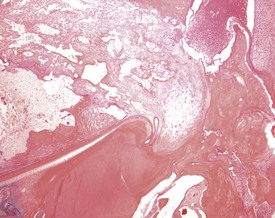
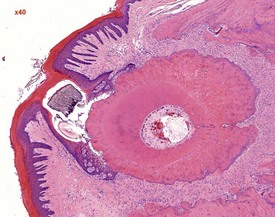
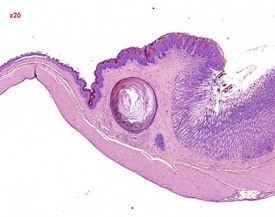
Dental dysplasia (Fig. 2.42) is a congenital lesion generally involving the incisor teeth and it may be related to malocclusion or food impaction (Fig. 2.43) (Monticello et al 1990). Dental dysplasia is the abnormal development of odontogenic tissues (Bertram et al 1996). Dental dysplasia is often observed in conjunction with inflammation and trauma. The lesion consists of a large mass of dentine-like material with fragments of tooth and bone (Bertram et al 1996). Cleft palate is a congenital lesion encountered in the midline of the hard palate of rats (Renne et al 2009). A pulp stone is a focal area of dentine in the pulp cavity which is often mineralized. Pulp stones often form around cellular debris (Bertram et al 1996).
Sebaceous gland ectopia in the oral cavity is observed at the base of the molar teeth (male rats) and between the upper incisors (Bertram et al 1996). This finding, referred to as Fordyce’s granules, which displays morphologically normal sebaceous glands, is described in several species including humans and rats. Histologically, Fordyce’s granules are identical to their cutaneous counterpart but are not associated with hair follicles.
In rats, there are, at present, only four reports of intraoral ‘ectopic’ sebaceous glands in the literature (Bernick & Bavetta 1962, Frandsen 1962, Rulli & Martinelli 1971, Yoshitomi et al 1990). In Holtzman and Long–Evans rats, as well as in Wistar rats, Fordyce’s granules are found in the molar gingiva. The most common site in the rat molar gingiva is probably adjacent to the first molar. Ultrastructurally, Fordyce’s granules consist primarily of typical lipid-filled sebaceous cells. In addition to the incisor and molar area, the buccal mucosa as well as the hard palate and tongue should also be considered as possible sites of occurrence of Fordyce’s granules in rats.
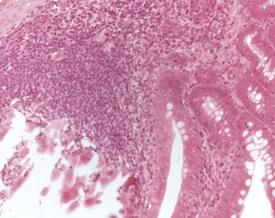
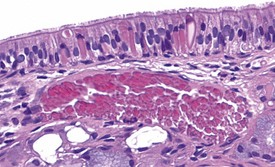
Squamous cysts (Fig. 2.44), often containing keratin, are common in the stomach, particularly in the region of the limiting ridge and antral mucosa. In addition, squamous cysts may also be encountered in the gingiva or associated with the teeth in the oral cavity (Bertram et al 1996) as well as in the esophagus (Bertram et al 1996). Glandular cysts (Fig. 2.45) lined by cuboidal or columnar epithelium may be observed in the glandular stomach (Bertram et al 1996). Megaesophagus is a congenital condition observed in young rats (Bertram et al 1996).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree