Chapter 8 Artifacts in histopathology
Introduction
What one may see upon microscopic examination of sections of animal tissues is not always related to the normal histology or pathology of the tissue in question. Defects or abnormalities in tissue sections may result from the faulty processing of the tissue specimens. These defects are referred to as artifacts. Some artifacts are easily distinguished from normal or pathological tissue components, and some are difficult to distinguish from such entities (McInnes 2005). This chapter will endeavour to illustrate some of the many hundreds of artifacts that are most frequently encountered in the preparation of microscopic tissue sections.
Before death/ante mortem
Several artifacts that are encountered in microscopic tissue sections are not the fault of histology technicians but have, in fact, been caused by clinical procedures performed ante mortem or by ante mortem environmental factors. Examples include the inclusion of suture material and carbon particles into tissues (Thompson & Luna 1978) as well as thermal and chemical dehydration. Thermal dehydration results from tissues removed by cautery, which causes coagulation of proteins and chemical dehydration results from the use of caustic chemicals to sterilize the surgical instruments. Carbon particles result from the inhalation of dust and are ingested by alveolar macrophages within the lungs. The condition is called arthracosis and is common in dog, non-human primate and human lungs.
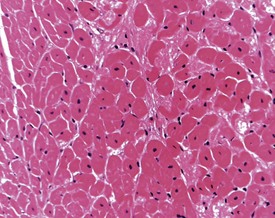
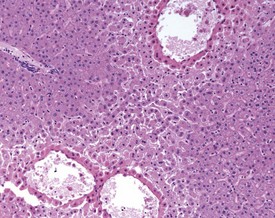
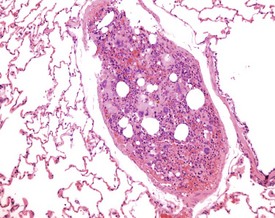
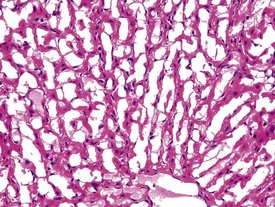
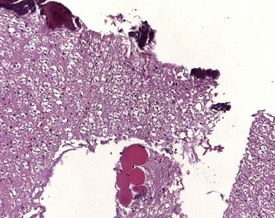
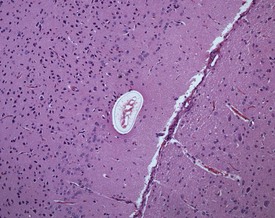
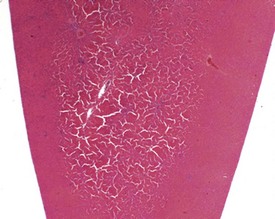
Although the hemorrhage and gliosis present within the optic nerve, caused by retro-orbital bleeding, represent a genuine pathological process, this lesion may still be considered to be an ante mortem artifact because it occurs regularly and can be confusing to an inexperienced pathologist (Fig. 8.1). In larger animals, euthanasia is generally achieved with intravenous barbiturate injection. Occasionally, the inability to find a suitable vein results in intracardiac barbiturate injection in order to cause rapid euthanasia. Barbiturate is highly caustic and when injected into the cardiac muscle it causes the intense eosinophilia of the cardiac myocytes as well as separation of shrunken eosinophilic fibres by a pink-staining amorphous material. The changes are considered to be artifactual and a result of barbiturate lysis (Darke et al 1996). This change may, however, be confused with severe degeneration or necrosis of the cardiac myocytes. The absence of concomitant inflammatory cells in the areas of eosinophilia militate against this diagnosis (Fig. 8.2). A similar lesion may be observed in the liver of monkeys euthanased with barbiturates. Here, the centrilobular hepatocytes demonstrate dilatation of the sinusoids and rarefaction of the hepatocytes (Fig. 8.3). Bone marrow biopsy before death under terminal anesthesia can result in bone marrow emboli lodging in the blood vessels of the lung (Fig. 8.4).
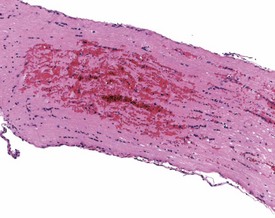
FIGURE 8.1 Haemorrhage and gliosis in optic nerve caused by retro-orbital bleeding in the rat. ×100.
Postmortem/necropsy
Numerous artifacts that are observed in microscopic tissue sections occur during the necropsy procedure. Examples of such artifacts include talcum powder inclusion, pressure effects, plant material, hair contaminants, bone fragment contamination and freezing artifacts (Thompson & Luna 1978). Talcum powder may become incorporated into the tissues at necropsy when powder contamination of the carcass occurs with the use of powdered latex gloves (Thompson & Luna 1978). Talcum powder is made up of starch and hydrous magnesium silicate. The powder appears as grey crystals in the processed tissues. Hair and grass may contaminate tissues at necropsy and may be observed in cross section in the processed slide (Fig. 8.5). In addition, it is important to note that autolysis (which is an artifact in itself) will start to occur within three minutes of death in the small intestine, resulting in the swelling of the villus tips and epithelial denudation of a few villi (Pearson & Logan 1978).
Tissues may be deliberately or accidentally frozen after death. Freezing artifacts (Fig. 8.6) are characterized by vacuolation and cracks caused by formation of ice crystals intracellularly and extracellularly due to freezing of the cadaver prior to necropsy examination. The ice crystals melt when the tissue is placed in fixative and this causes cell rupture and the formation of spaces within the interstitium (Thompson & Luna 1978). Softer tissues containing greater amounts of fluid are more vulnerable to freezing artifacts than are harder tissues such as smooth muscle (Thompson & Luna 1978). Freezing artifacts may also occur when tissues are frozen after placement into fixative, usually when transported in freezing weather with inadequate insulation (Thompson & Luna 1978).
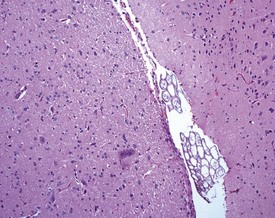
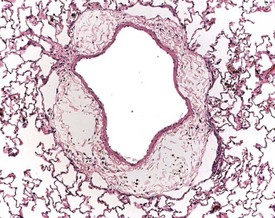
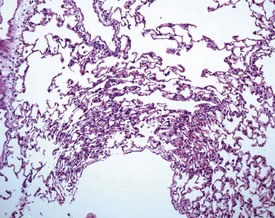
Perfusion of the lungs with buffered formalin at necropsy is an accepted technique to improve the histology of the pulmonary architecture (Hausmann et al 2004). This may result in a lesion that resembles perivascular edema (Fig. 8.7). Failure to perfuse lung tissue at necropsy results in collapse of the interstitial, alveolar tissue and the lung may appear to demonstrate pneumonia. Pressure exerted on tissues by forceps (Fig. 8.8) at necropsy may result in ‘pinch’ defects which do not resolve during processing (Thompson & Luna 1978). Such artifacts cannot be corrected during processing of the tissue specimen; however, they can be prevented by solicitous handling of fresh tissue specimens prior to fixation. Fragments of bone are inserted commonly deep into the brain tissue (Fig. 8.9), due to carelessness on the part of the prosector, during the opening of the cranium to remove the brain. Such fragments of non-decalcified bone are extremely hard in comparison with the surrounding soft brain tissue. In addition to nicking the edge of the microtome knife, the bone fragments are moved by the microtome knife-edge during cutting and this causes shattering and distortion of the tissue section (Thompson & Luna 1978).
Tissue may become contaminated with fragments of the animal’s hair (Fig. 8.10) at the time of necropsy and prior to fixation. Such surface contaminants are often not removed by washing the tissue specimens subsequent to fixation and prior to further processing, although this is the only method of removal. In some cases, depending on the orientation of the shaft of the hair, the knife can push the hair or bone further into the tissue and produce shattering of softer tissue (Thompson & Luna 1978). Suture material may also become embedded in tissues, and a similar effect is caused when suture material is pushed ahead of the microtome knife and causes shattering of the tissue adjacent to the site of localization (Thompson & Luna 1978).
Fixation
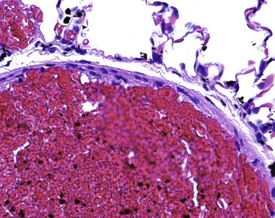
Many of the artifacts observed in microscopic tissue sections are the result of incorrect fixation procedures. Examples of fixation artifacts include the use of an incorrect fixative for a particular tissue specimen and the formation of acid formalin haematin pigment. In addition, autolytic changes due to specimen adherence to the inner surface of the fixative container or the use of inadequate quantities of fixative may also occur. The practice of placing tissues in a container and then adding the fixative often results in the adherence of the tissue to the sides of the container, with consequent autolysis of the adherent surface of the tissue. Thick tissue specimens, or insufficient time spent in fixative, may result in a similar artifact with a focal area of autolysis visible in the middle of the tissue (Fig. 8.11). Cracking in the centre of tissues may occur due to inadequate fixation in formalin. When the tissue is processed, the central unfixed tissue is effectively fixed with ethanol, and shrinks, and thus forms more cracks than the peripheral formalin-fixed tissue during subsequent processing (Fig. 8.12).
FIGURE 8.11 Central areas of pink autolytic tissue in a liver specimen due to inadequate penetration of fixative.
Biopsy specimens are often not fixed for a sufficient length of time. Fixation requires at least 48 hours and a change of fixative at 24 hours is recommended. Formalin penetrates tissues at a rate of 16 to 28 mm per 24 hours. If the tissues are thicker than 6 mm, then longer fixation is recommended. The maximum thickness of a specimen should not exceed 6 mm, and for each volume of tissue, 20 volumes of fixative should be used (Thompson & Luna 1978). Saprophytic bacteria may occur in areas of autolysis. The saprophytic bacteria are basophilic and are not accompanied by inflammatory cells when the tissue is examined under the microscope. In addition, gas bubbles, due to the presence of clostridial bacteria, may also be observed in autolysed tissues.
Black, amorphous crystals may result from fixing in mercuric chloride–formaldehyde (this artifact is not very common today due to the toxicity of mercuric chloride and its subsequent disuse). The mercuric chloride artifact may be removed with iodine and sodium thiosulphate. Contamination of the specimen with rust (formed as a result of oxidation of the metal cap covering the formalin container) is another artifact which may occur in histopathological tissues (Thompson & Luna 1978).
Acid formalin haematin pigment is a dark brown, anisotropic, microcrystalline, iron-negative pigment (Fig. 8.13). It is produced by the reaction of formic acid from unbuffered formalin with the heme of hemoglobin at an acid pH, and it can be removed with a saturated alcoholic picric acid solution or by fixation in phenol–formalin (Thompson & Luna 1978). This pigment results in a brown formalin pigment in, on, or around erythrocytes within tissue sections. Consequently the pigment may be encountered in a wide variety of sites within tissue specimens because these sites may contain heme. In tissue sections, a black to brown, amorphous to microcrystalline pigment is observed particularly over blood vessels and it is often not in the plane of section. In the kidney, formalin pigment tends to accumulate in glomeruli.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree