Chapter 9 Reproduction of the rat, mouse, dog, non-human primate and minipig
Reproduction: male
Male reproductive tract
Recognising normal spermatogenesis in the testis
In the male animal, recognising the different cellular associations that make up the spermatogenic cycle within seminiferous tubules is essential to be able to identify when cell populations are missing or, in the case of spermatid retention, when they are inappropriately present. An additional challenge in the male animal is the problem of recognising immaturity and distinguishing it from degenerative conditions. This is a particular problem in the dog and non-human primate. Spermatogenesis staging is aided by the use of the correct fixative to preserve the testes (Latendresse et al 2002; Lanning et al 2002).
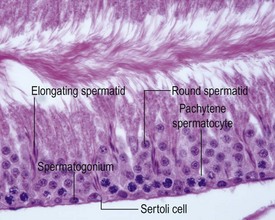
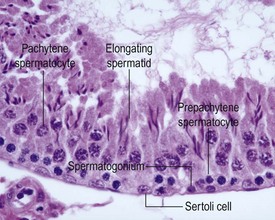
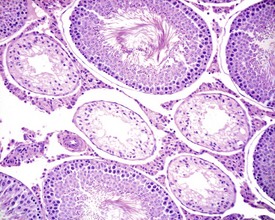
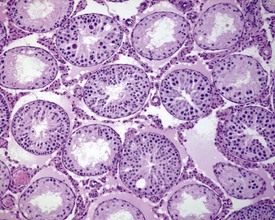
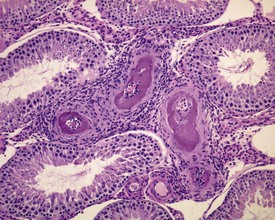
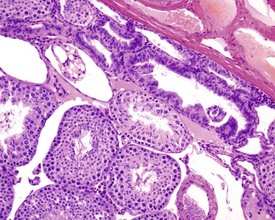
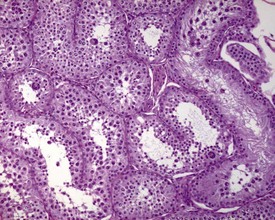
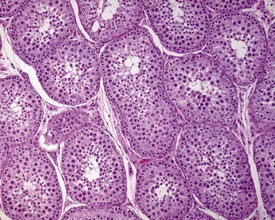
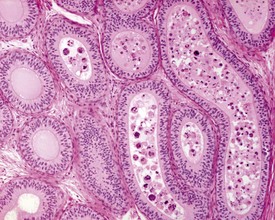
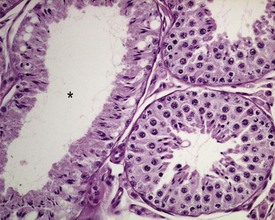
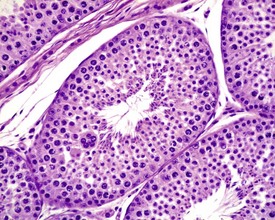
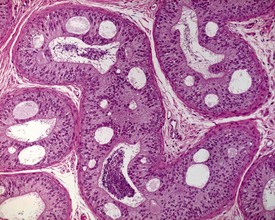
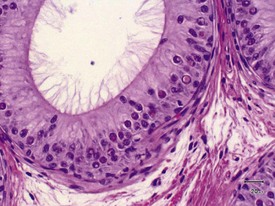
The different types of germ cell (spermatogonia, spermatocytes and spermatids) are arranged in a very regular, layered pattern within the seminiferous tubules (Figs 9.1, 9.2). They are supported both structurally and metabolically by the somatic Sertoli cell, which entirely surrounds each germ cell with cytoplasmic processes not readily visible by light microscopy. The true extent of the Sertoli cell becomes more apparent when germ cells are lost from the tubule, leaving only Sertoli cells lining the tubules (Fig. 9.4).
The germ cells proceed through spermatogenesis in a highly controlled manner, and in any given tubule there are always four generations of germ cells developing in complete synchrony with one another. This gives rise to the concept of stages of the spermatogenic cycle, where each stage can be identified by the precise morphological characteristics of the individual germ cells from the four synchronously developing generations. The detailed description of the cell associations is beyond the scope of this chapter but is comprehensively covered in a number of reviews (Creasy 1997; Creasy & Foster 2002; Russell et al 1990). It is important that the toxicological pathologist be familiar with the concept of the spermatogenic cycle and the different appearances of tubules, at least at the beginning, middle and end of the cycle (Figs 9.1, 9.2) (Foley 2001).
Rat and mouse
Immaturity, peripuberty, maturity
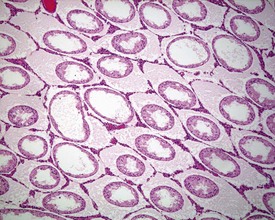
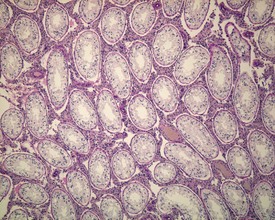
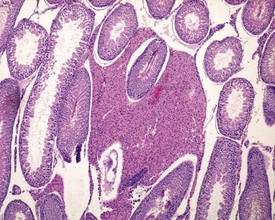
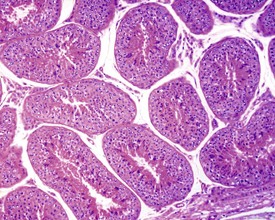
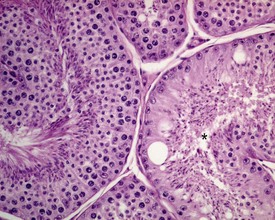
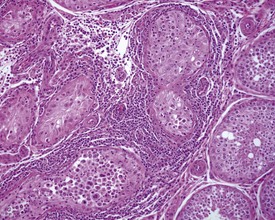
Rats start releasing sperm from the testis at about six to seven weeks of age, but sperm output does not reach its full potential until about 10–11 weeks of age (for review see Marty et al 2003). The first cycle of spermatogenesis is relatively inefficient, with reduced numbers of maturation-phase spermatids and numerous degenerating germ cells (multinucleate and apoptotic cells) in the seminiferous tubules. The cauda epididymis is only partially expanded and contains variable numbers of sperm as well as cell debris and degenerating sloughed germ cells from the testes (Fig. 9.3a & b). Not all rats will mature at the same rate, and so the appearance can easily be mistaken for testicular toxicity, especially if the test-article-treated animals are slightly less sexually mature due to decreased food intake or decreased body weight.
Lesions seen in young animals: testes
Tubular degeneration/atrophy
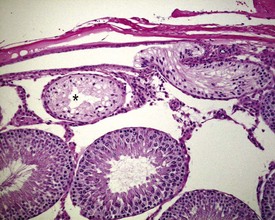
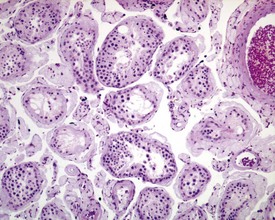
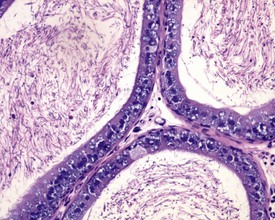
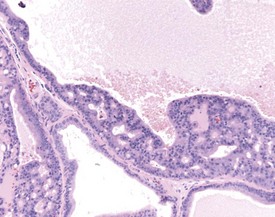
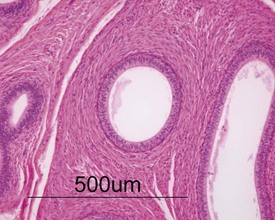
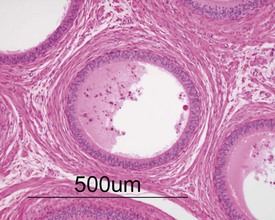
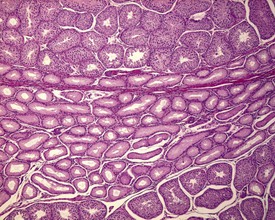
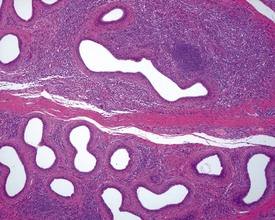
Occasional tubules (five tubular profiles/testis) with partial or complete depletion of germ cells is a common finding in young adult rats (Fig. 9.4). Tubuli recti, which are the transitional tubules that connect the seminiferous tubules with the rete testis, should not be mistaken for atrophic tubules. Tubuli recti are generally seen immediately adjacent to the main rete testis lumen that is situated at the cranial pole of the testis (Fig. 9.5). The tubuli may also be present in a more lateral subcapsular position, because fingerlike extensions of the rete radiate out from the main sac-like structure.
More extensive tubular atrophy, often involving complete loss of all germ cells from all tubules in one or both testes, can sometimes be seen as a background finding in young adult rats (Fig. 9.6a). If the atrophy has been present for more than a few weeks the corresponding epididymis will probably be depleted of sperm and/or germ cells. This can be a useful indication of whether the atrophy was pre-existing, prior to study start, and therefore a background change. Moderate to severe tubular atrophy is often accompanied by increased interstitial fluid (sometimes termed edema) (Fig. 9.6b). Care should be taken when using this diagnosis because fluid accumulation can occur as a fixation artefact, particularly in Bouins-fixed testes (Fig. 9.6b).
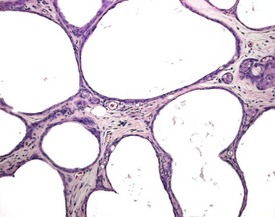
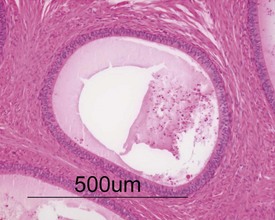
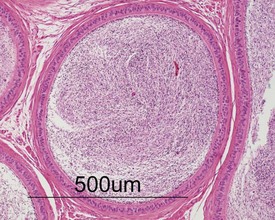
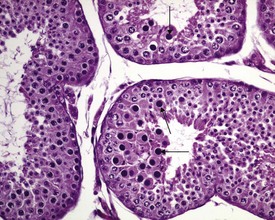
Other findings that can occasionally be seen include tubular dilatation (unilateral or bilateral) (Fig. 9.7) which may be associated with a dilated rete. Tubular degeneration characterized by varying numbers of degenerating germ cells, including multinucleate giant cells and/or disorganisation of germ cell layers, is sometimes seen as a bilateral background finding (Fig. 9.8).
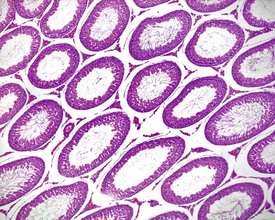
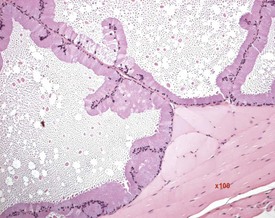
FIGURE 9.7 Unilateral or bilateral tubular luminal dilatation is occasionally seen in young adult rats. The affected testis or testes are enlarged and generally show increased weight. The lesion may be associated with a dilated rete and likely reflects fluid build-up due to obstruction of the efferent ducts. The lesion generally progresses rapidly to complete tubular atrophy (as in FIGURE 9.6a) due to pressure atrophy, but the tubular lumina often remain dilated. ×100.
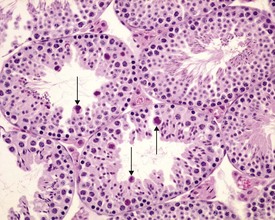
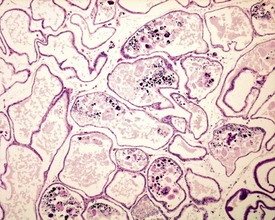
Abnormal residual bodies (Fig. 9.9) are most commonly seen in the mouse and are characterized by the presence of abnormally large and dense residual bodies that are usually located on the luminal surface of the germinal epithelium in tubules at various stages of the spermatogenic cycle. In contrast, normal residual bodies are much smaller and should only be present at the luminal surface of stage VIII–IX tubules, following which they rapidly descend to the basal region of stage X–XI tubules where they are phagocytosed. Abnormal residual bodies are often seen in tubules in other stages of the cycle.
Lesions seen in young animals: epididymides
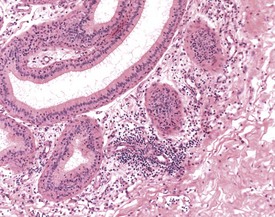
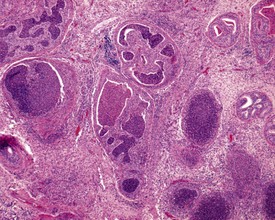
Sperm granulomas are one of the most common changes seen in young rat epididymides. They may be unilateral or bilateral and can occur anywhere in the epididymis, but are more commonly noted in the cauda (Fig. 9.10). Occasional lymphoid aggregates in the interstitium of the epididymis are a common finding, but are generally few in number.
Cribriform change, characterized by intra-epithelial microcysts, is a common finding at the junction of the corpus/cauda (Fig. 9.11).
Age-related lesions: testes and epididymides
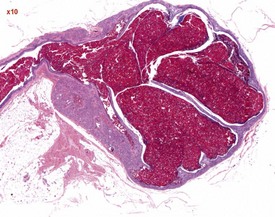
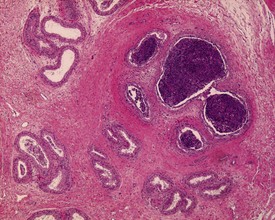
The most common age-related testicular findings include degenerative and inflammatory lesions of the vasculature, including fibrinoid necrosis, hypertrophy and inflammation of the vascular wall as part of the more generalized condition of polyarteritis nodosa (Fig. 9.12a). Lymphoid aggregates within the interstitium of the rat epididymis are common (Fig. 9.12b). The testis is also a common site for deposition of amyloid in systemic amyloidosis of the aging mouse (Fig. 9.13). Focal proliferative changes in the Leydig cell population (hyperplasia and tumors) (Fig. 9.14) are very common in the F344 rat, and are also present at a lower incidence in other strains of rats and mice. Another common proliferative lesion in the mouse testis is hyperplasia of the rete epithelium (Fig. 9.15). Lipofuscin pigmentation is associated with aging and is observed in the interstitial macrophages and Leydig cells.
In the aging rat, the segment of the epididymis at the junction between the caput and corpus commonly develops a foamy, basophilic vacuolation within the epithelium (Fig. 9.16). This probably represents the normal glycoprotein secretory product that has become modified in the aging rat. If the cystic rete testis is filled with spermatozoa, the lesion is referred to as a spermatocoele. Spermatocoeles are also common at the junction between the epididymis and vas deferens.
Age-related lesions: accessory sex organs and penis
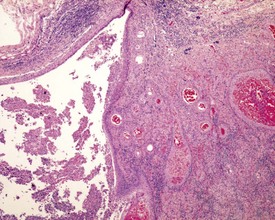
Urogenital infections affecting the penis, preputial glands, prostate, coagulating glands and seminal vesicles are common in aging rodents, particularly in mice (Suwa et al 2001). Acute or chronic active inflammation with abscess formation (Figs 9.17a, 9.18) may be present throughout the genital tract (Fig. 9.17a). Focal epithelial hyperplasia is observed in the aging rat prostate (Fig. 9.17b).
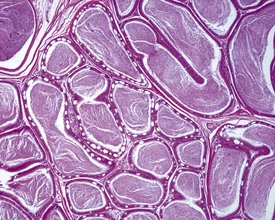
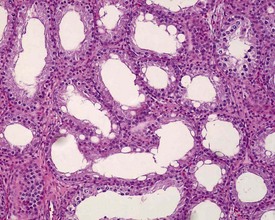
In the accessory sex organs, common age-related lesions include distension of the seminal vesicles with degradation of the seminal fluid (Fig. 9.19) or contraction of the vesicles with atrophy of the epithelium. The prostate also shows progressive acinar atrophy and mineralized concretions in the acinar lumina (Fig. 9.20). Cystic dilatation of the preputial gland ducts with secretion or cystic atrophy of the acini are common findings in both rats and mice (Figs 9.21, 9.22). Similar cystic dilation can also be seen in the bulbourethral gland (Fig. 9.23).
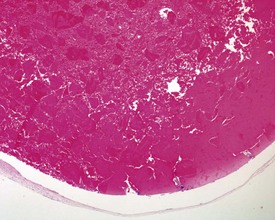
FIGURE 9.19 Grossly distended seminal vesicle of a mouse with degradation of the secretory product. ×100.
Dog
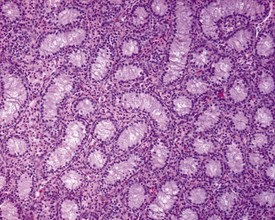
Background changes in Beagle dog testes are common and may obfuscate chemically-induced toxicity. Spermatogenesis in the dog appears to be much less efficient than that in the rodent or non-human primate and seminiferous tubules frequently show incomplete spermatogenesis (hypospermatogenesis). One of the greatest problems identifying chemically-induced toxicity is caused by using dogs that are still sexually immature or peripubertal at the time of examination. It is therefore important that the pathologist understands the characteristics of immature, peripubertal and mature reproductive tissues so that the degenerative changes that characterize this period of morphological development are not mistaken for toxicologically-induced disruption of spermatogenesis. The normal appearance of spermatogenesis in the dog testis and the cell associations of the stages of the spermatogenic cycle have been described in detail by Russell and coworkers (1990).
Immaturity, peripuberty, maturity
Dogs mature over a relatively wide age range (seven to twelve months of age), with most reaching sexual maturity by ten months of age. The age of sexual maturity is also influenced by the supplier. Dorso and coworkers (2008) reported that 90% of dogs 31–40 weeks of age supplied by Harlan, France, were sexually mature compared with only 10% of dogs of the same age supplied by Marshall Farms, USA. Dogs less than ten months of age usually show a range of degenerative morphologies reflecting ongoing maturation. Dogs on the borderline of maturity will have varying numbers of degenerating, missing and sloughed germ cells in the testis and the epididymal lumina and some tubules may be vacuolated, contracted or dilated. The appearance of the testes and epididymal contents can be indistinguishable from chemically-induced toxicity, and evaluating the relationship of such changes to treatment is made more difficult by the small number of animals in each group. The confusion can be avoided by ensuring that dogs are at least ten months and preferably 12 months of age at the end of the study. The morphological characteristics of the developing dog testis and epididymis are illustrated in Figs 9–.24-9.29 and Figs 9.30–9.34 respectively.
Unlike the rodent, the dog goes through a developmentally quiescent period with respect to testicular development. During this period, the seminiferous tubules are lined by Sertoli cells and small numbers of gonocytes (pre-spermatogonia) which do not begin development until around five months of age (Fig. 9.24). At that time the gonocytes begin to proliferate and produce spermatogonia which will populate the basal layer of the seminiferous tubule and initiate the process of spermatogenesis. At the same time, the Sertoli cell resumes its maturation and begins to secrete seminiferous tubule fluid. This often takes the form of fluid-filled vacuoles within the layer of Sertoli cells and the gradual formation of a tubular lumen and expansion of the overall tubular diameter (Figs 9.25, 9.26). Spermatogonia begin to proliferate and produce spermatocytes, which in turn divide and produce round spermatids. The round spermatids gradually elongate and mature into the final whip-like mature spermatids, which are released into the tubular lumina. From there they are transported on into the epididymis. Spermatogenesis does not develop at the same rate in all tubules, and so different tubules will be more or less advanced than others and because the Sertoli and germ cells are not yet up to full functional efficiency, there will be considerable degeneration and sloughing of germ cells in this first cycle of spermatogenesis (Fig. 9.27). The gradual increase in seminiferous tubule fluid production and the high rate of germ cell attrition is reflected by the luminal contents of the epididymis (Figs 9.30–34). Small-diameter, empty ducts will gradually expand with fluid and contain degenerate sloughed germ cells through to puberty (Fig. 9.34), to be replaced by expanded ducts filled with mature sperm and relatively few degenerate cells in the fully mature adult (Fig. 9.33).
Lesions seen in young mature animals: testes
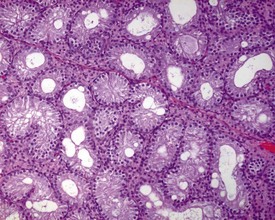
Changes that can easily be confused with immaturity, but which are background degenerative changes of the sexually mature dog, are seen commonly in dogs that are more than 10 months of age. The changes have been described by Rehm and coworkers (2000) and by Goedken and coworkers (2008). The most common findings are tubular hypospermatogenesis and tubular hypoplasia/atrophy, both of which have an approximately 30% incidence in normal dogs. Hypospermatogenesis is characterized by the absence of one or more generations of germ cells from the seminiferous epithelium (Figs 9.35, 9.36). Hypospermatogenesis may be distinguished from immaturity by the fact that many of the tubules will have a layer of mature, elongate spermatids at the luminal surface, but with the underlying layer of round spermatids and/or layer of pachytene spermatocytes missing or partially depleted. This is indicative of transient (cyclical) inhibition of spermatogonial division and is different from the pattern of germ cell degeneration and patchy germ cell depletion typical of immaturity (compare with Fig. 9.27). Hypospermatogenesis can range in severity from minimal (<10% tubules affected) to severe (>75% tubules affected). Most testes will have a few tubules affected by hypospermatogenesis.
Segmental hypoplasia is typically seen as a discrete group of contracted tubules, often in a wedge shape and situated in a subcapsular location, which are completely devoid of germ cells (Fig. 9.37). These tubules give the appearance of never having been populated by germ cells, hence the term hypoplasia. The lobular distribution suggests that the group of tubular profiles belong to a single, coiled, seminiferous tubule.
Other common background findings in the adult dog testis include swollen spermatocytes. These are probably secondary spermatocytes which have failed to complete their second meiotic division and remain sequestered in the germinal epithelium, arrested in meiosis, while the surrounding cells continue their development into round spermatids (Fig. 9.38). Occasional multinucleate germ cells, which represent degenerating spermatocytes or spermatids, are also a common feature of dog testes (Fig. 9.39). Both findings can usually be seen in a few tubules from most dog testes.
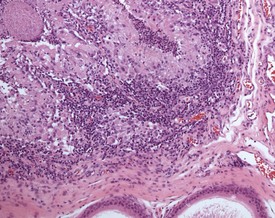
Orchitis, characterized by a lymphocytic or mixed inflammatory cell infiltrate in the interstitium of the testis, may occasionally be seen in the dog testes (Fig. 9.40) (Fritz et al 1976). The inflammation may also extend into the tubular epithelium, resulting in destruction of the affected tubules. The lesion is generally accompanied by a lymphocytic inflammation in the epididymis (Fig. 9.41) and in some cases is genetically associated with lymphocytic thyroiditis (Fritz et al 1976). The lesion is thought to have an autoimmune etiology.
Lesions observed in young mature animals: testes
Sperm stasis, sometimes accompanied by inflammation, is a common finding in the efferent ducts situated in the head of the epididymis, and probably represents impaction of sperm in blind-ending efferent ducts (Fig. 9.42) (Foley et al 1995). True sperm granulomas, similar to those seen in rodents, also occur throughout the epididymis.
Cribriform change, characterized by infolding of the ductal epithelium with the formation of pseudoglandular cysts (Fig. 9.43), is often observed at the corpus/caudal junction. This is similar to the lesion in rodents. Intranuclear eosinophilic inclusion bodies are a feature of the canine epididymis (Fig. 9.44), particularly in the caput, although their significance is unknown.
Interstitial lymphocytic infiltrates are common and may also be accompanied by arteritis or periarteritis either in the interstitium or in the surrounding adipose tissue of the dog epididymis. This is a common site for arteritis associated with the more generalized condition of ‘beagle pain syndrome’. Epithelial hyperplasia of the ductus epididymis is a normal feature of the aged beagle dog epididymis (James & Heywood 1979).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree