Chapter 21 Wildlife Disease Ecology
What Can Zoo and Wildlife Veterinarians Learn from this Discipline?
History of Development of Wildlife Disease Ecology
With few exceptions, the field of ecology largely ignored disease and its effect on populations, communities, and ecosystems until fairly recently; this only changed as a result of mathematical models designed by May and Anderson in the 1970s, which were syntheses between parasitology and population biology.2 These earlier models were significant because they presented the effect of pathogens on hosts and populations as another density-dependent mechanism driving populations toward equilibrium or keeping populations from exponential growth, and were analogous to predator-prey models. Disease ecology focuses on understanding how infectious diseases spread through and affect host populations and how hosts and pathogens react and evolve in response to one another; thus, it borrows principles from epidemiology and evolutionary biology. More recent events that advanced disease ecology forward were the Isaac Newton Institute meeting which resulted in one of the first disease ecology texts, soon followed by others such as The Ecology of Wildlife Diseases,12 Disease Ecology: Community Structure and Pathogen Dynamics3 and, most recently, Infectious Disease Ecology: Effects of Ecosystems on Disease and of Disease on Ecosystems.21 With the development of each text, the theory of disease ecology has been increasingly supported by empirical data with applied objectives.
Principles of Disease Ecology
as illustrated in Figure 21-1.23 These equations may then be rearranged to determine the threshold value of infection.
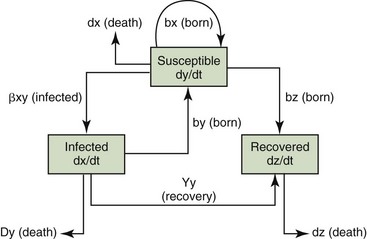
Figure 21-1 Relationships among susceptible, infected, and recovered individuals in an infection model.
(Modified from Ricklefs RE, Miller GL: Ecology, ed 4, New York, 2000, WH Freeman.)
Significance of Disease Ecology for Zoo and Wildlife Veterinarians
Most zoo and wildlife veterinarians are involved in some aspect of disease investigation. Wildlife disease investigation developed firstly as a descriptive science—the who, what, where, when and why of epidemiology—as a response to a need for management, particularly in response to public health concerns.28 Most wildlife disease investigation stems from visible mortality events, or epizootics. Wildlife disease specialists are traditionally trained through veterinary medicine and/or master’s or doctoral programs in wildlife disease, pathology, virology, microbiology, or related sciences. These disciplines, when applied to the field of wildlife disease investigation, adhere strictly to the principles of epidemiology. More recently, wildlife disease investigation has become more experimental in nature. Ultimately, epidemiologists and wildlife disease specialists share the common goal of limiting or managing diseases, which sometimes moves beyond concentrating on the host-pathogen situation and involves environmental modifications. Currently, however, wildlife disease investigation has also grown to involve questions that bring it more closely to disease ecology, such as the influence of disease on population dynamics, the effects of chronic and more subtle diseases on the fitness of hosts, and the effects of diseases on small isolated populations (what we call the “so what”). It is this latter stretching of traditional epidemiology and merging with the principles of wildlife biology and ecology that comprise the future of the science of wildlife disease investigation. For the last 10 years, the number of presentations dealing with pathogens and disease at the annual conference of the Ecological Society of America has grown exponentially and, equally, disease ecology sessions have been found at the Wildlife Disease Association meetings consistently for the last 5 years. At the center of these two disciplines, traditional wildlife disease investigation and wildlife disease ecology, stands conservation medicine. The American Association of Zoo Veterinarians and the Wildlife Disease Association have both had sessions and journal sections consistently dedicated specifically to conservation medicine for the last 5 to 8 years. Conservation medicine, truly multidisciplinary, offers both groups a common ground of interest and is a field to which both zoo and wildlife veterinarians are actively contributing.
Therefore, we strongly believe that any veterinarian involved in wildlife disease investigation and conservation medicine will greatly benefit from understanding basic disease ecology principles and from reviewing the body of literature that this discipline has to offer. Given the ease with which literature searches are now available, it is logistically simple to peruse a number of disease-related publications.* To save time and effort, busy veterinarians may use automated online search and alert systems such as PubCrawler, a free alerting service that scans daily updates to the PubMed and GenBank databases and alerts users of the current contents of Medline and GenBank by listing new articles that match their customized research interests.
Population Effects of Disease
*These include the Journal of Ecology, Frontiers in Ecology, Ecological Applications, Ecological Monographs, Ecology Letters, Journal of Animal Ecology, Public Library of Science (PLOS) Biology, PLoS ONE, Journal of Applied Ecology, Emerging Infectious Diseases, Proceedings of the Royal Society of London B (Biology), Conservation Biology, Proceedings of the National Academy of Sciences of the United States of America, and EcoHealth.
Veterinarians are well trained in recognizing, identifying, diagnosing, and treating diseases in individual animals. Although herd health is paramount to some aspects of veterinary medicine, the potential population effects of pathogens are at the core of disease ecology because they may explain some cycling in population numbers. For example, one of the best-recognized empirical studies demonstrating population cycles driven by disease was by Dobson and Hudson4 on red grouse (Lagopus lagopus scoticus) in northern England, whereby infection with Trichostrongylus tenuis was shown to have negative effects on grouse fecundity, leading to population cycles that were not explained by other factors such as climatic effects or food availability. This is a simple but elegant article detailing the experimental treatment of isolated red grouse populations with antiparasitics, which demonstrated the significant attenuation of grouse population cycles in treated grouse and put the effects of parasites on populations on the map.11
The science of ecology has explored the effects of parasites and pathogens on host populations in other ways, such as through the hypothesis that assumes that parasites alter male secondary sexual characteristics and that females choose less parasitized males as a way to select males that may have resistance genes that can be passed on to her offspring.23 Again, experiments with a lekking species, the sage grouse (Centrocercus spp.), were able to demonstrate that males infected with avian malaria attended less frequently, bred later in the breeding season than healthy males, and mated with less fit females.13 Ecologic studies have also been useful to tease out complexities such as the relationship between environmental factors and infection, expression of clinical disease, or severity of disease in populations. The expression of clinical mycoplasmosis in desert tortoises and mortalities of lions caused by coinfection with canine distemper and Babesia spp. positively correlated with periods of drought illustrate these complexities. Additionally, chicks of sage thrashers (Oreoscoptes montanus) that were parasitized with blowfly larvae grew and fledged at the same rate as nonparasitized chicks during years with average precipitation, whereas significant differences in offspring survival and fledging success occurred in years with adverse climatic conditions.10 These subtleties in the effects of pathogens on individuals that are translated to population effects are largely being examined by disease ecologists and will likely revolutionize our thinking about disease in wild animals.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree