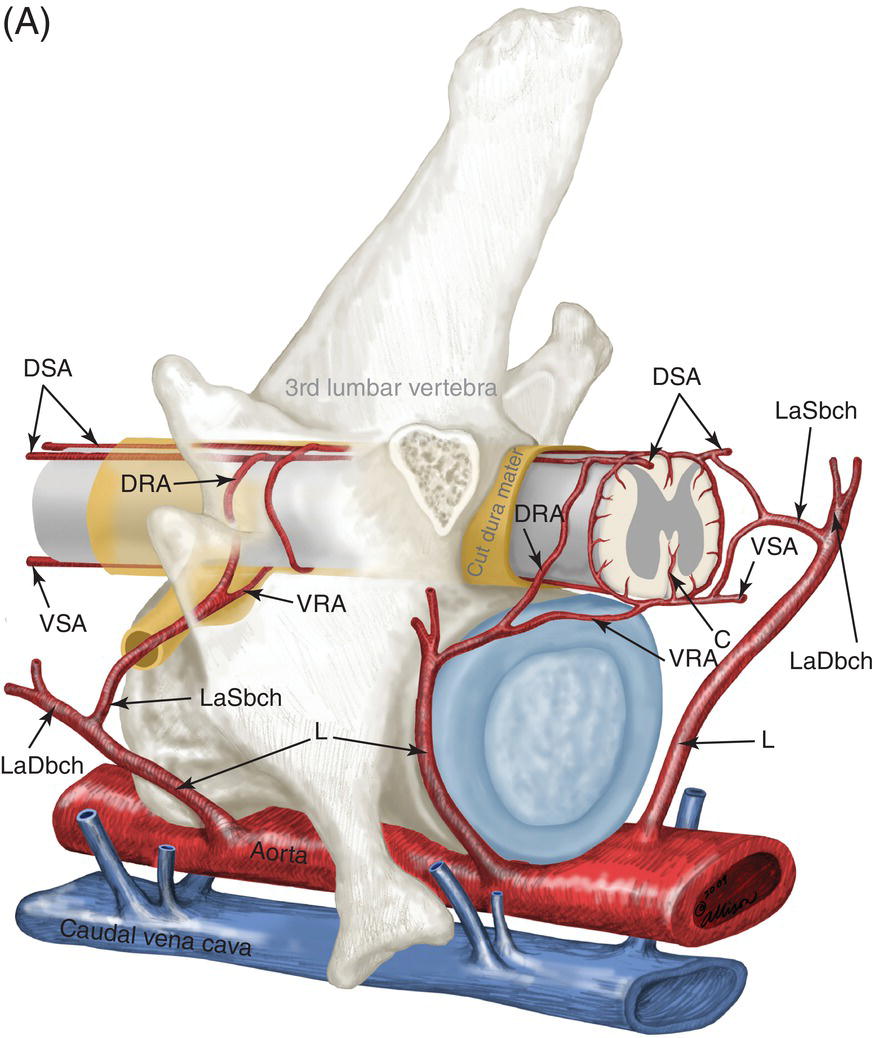
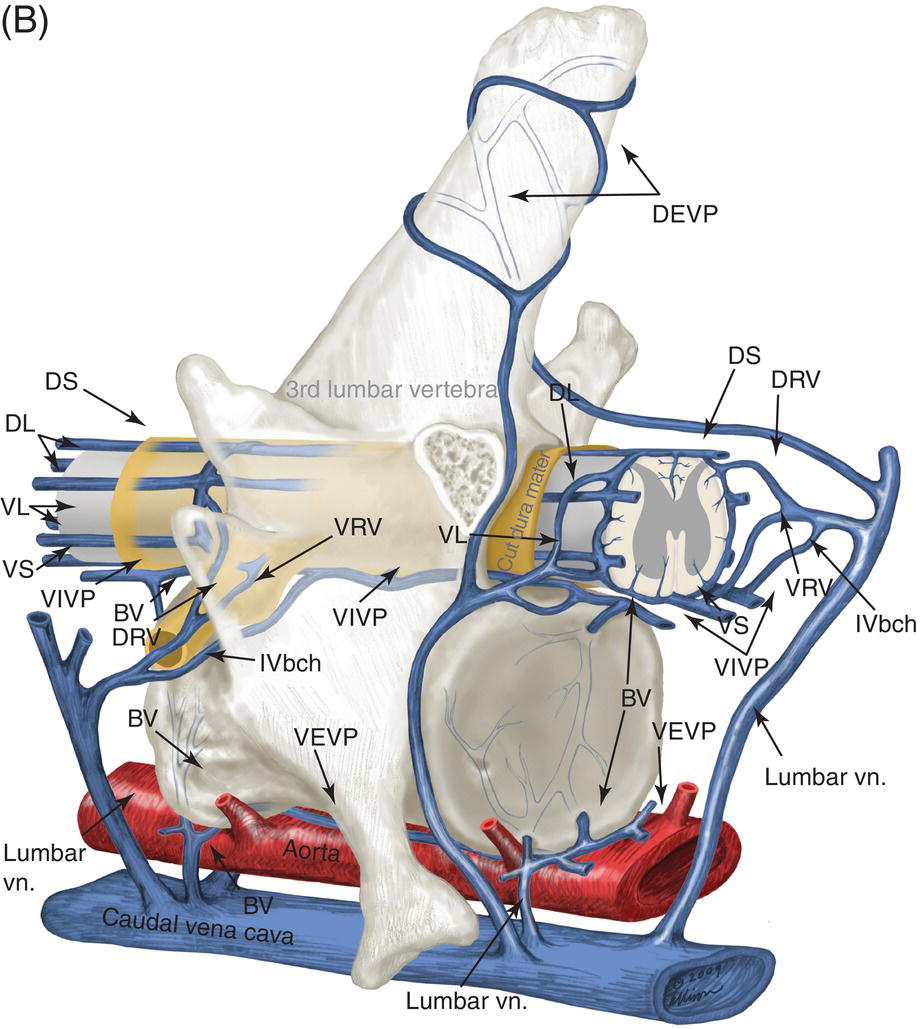
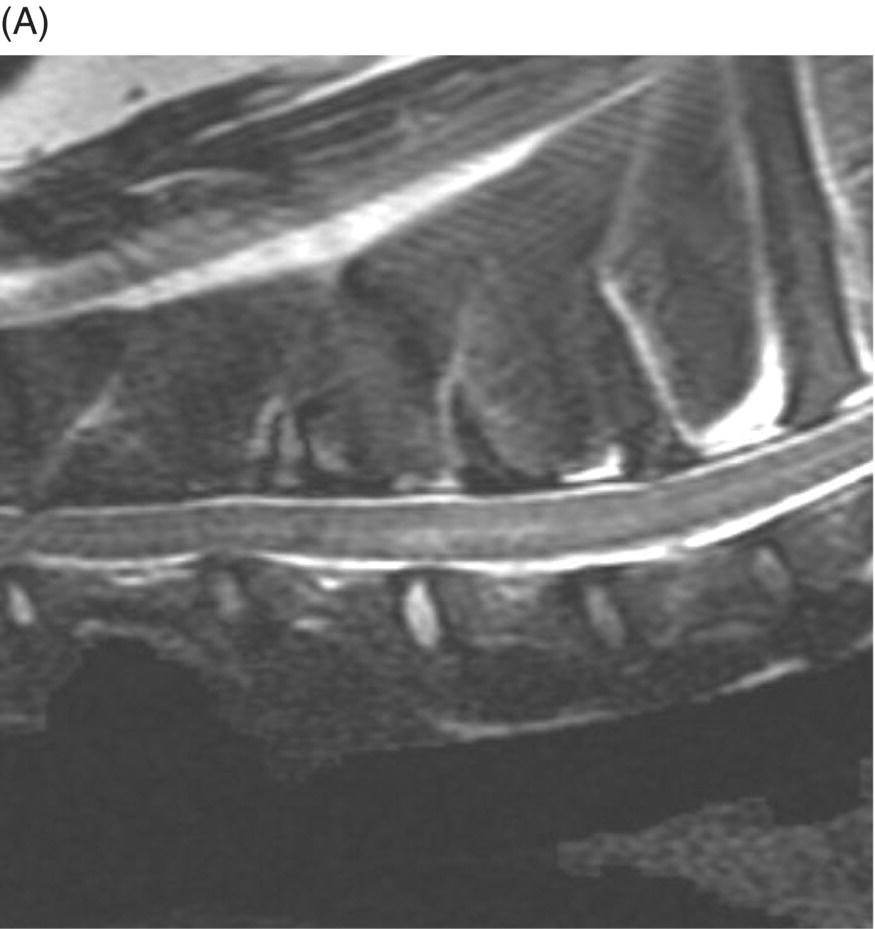
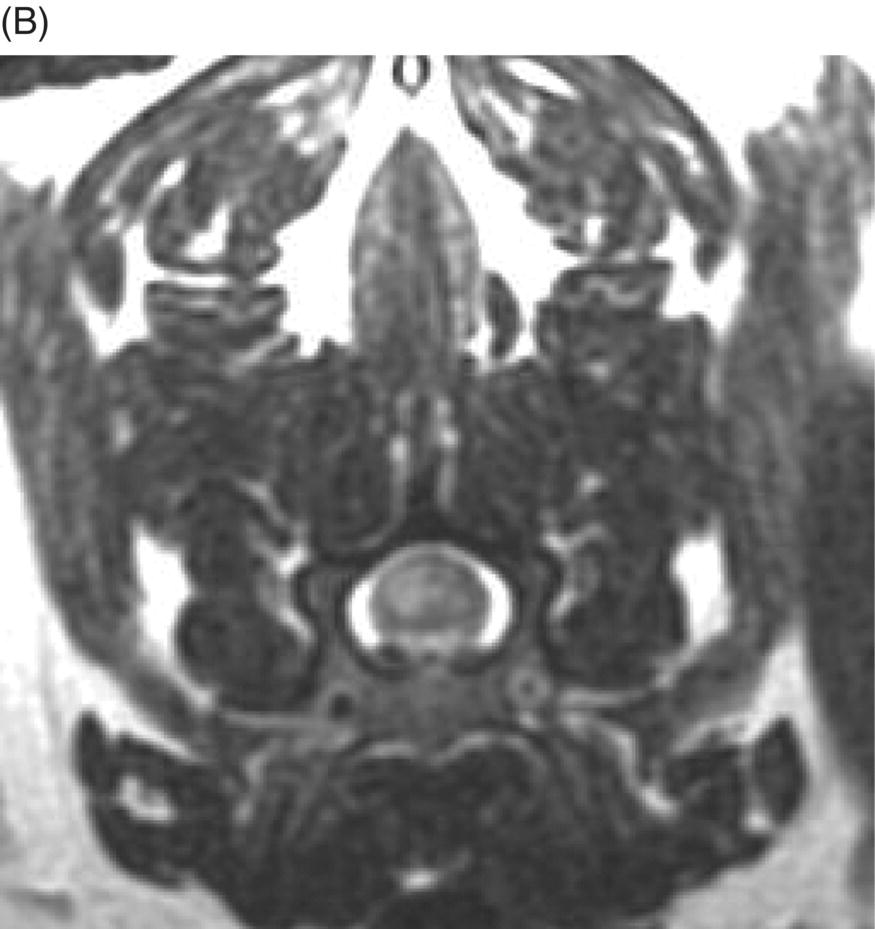
9 Luisa De Risio Embolization of spinal vasculature with fibrocartilaginous material histochemically identical to the nucleus pulposus of the intervertebral disc is the most common cause of ischemic myelopathy in dogs and cats. This condition is called “fibrocartilaginous embolism” (FCE) or “fibrocartilaginous embolic myelopathy” (FCEM). The intraparenchymal (intrinsic) spinal cord arteries are functional end arteries and their occlusion results in ischemic necrosis of the territory supplied. This typically results in peracute (<6 h) onset of neurological signs, with distribution and severity referable to the site and extent of the infarction. Neurologic deficits typically do not progress beyond the first 24 h. The source of the fibrocartilaginous material is assumed to be the nucleus pulposus that is undergoing degeneration in adult animals. This hypothesis is supported by the observation that FCE occurs primarily in the spinal cord and the lesion commonly extends over a few spinal cord segments, suggesting that the emboli arise from one degenerated intervertebral disc. The intervertebral disc is normally avascular in adults. Exactly how the fibrocartilaginous material gains access to the spinal cord vasculature is not known, but several theories have been proposed [1, 2]. These include: In immature animals, the annulus fibrosus is vascularized, and the semiliquid nucleus pulposus could be injected in the annulus fibrosus blood vessels when the intradiscal pressure suddenly increases during exercise or minor trauma [6]. The vertebral growth-plate cartilage (which is vascularized) could also be the source of the fibrocartilaginous material in immature dogs [7]. Another hypothesis on the source of the fibrocartilaginous material suggests that it may arise from metaplasia of the vascular endothelium, which later ruptures into the lumen and embolizes within the intrinsic spinal cord vasculature [1]. The vascular anatomy of the spinal cord is illustrated in Figure 9.1. Figure 9.1 (A) Arterial supply to the lumbar spinal cord. C, central artery; DRA, dorsal radicular artery; DSA, dorsal spinal artery (paired); L, lumbar artery; LaDbch, lumbar artery dorsal branch; LaSbch, lumbar artery spinal branch; VSA, ventral spinal artery; VR, ventral radicular artery. (B) Venous drainage of the lumbar spinal cord. BV, basivertebral vein; DEVP, dorsal external vertebral venous plexus; DL, dorsolateral vein (paired); DRV, dorsal radicular vein; DS, dorsal spinal vein; IVbch, intervertebral vein branch; VEVP, ventral external vertebral venous plexus; VIVP, ventral internal vertebral venous plexus; VL, ventrolateral vein (paired); VRV, ventral radicular vein; VS, ventral spinal vein. Source: With permission from De Risio L, Platt SR. 2010. Fibrocartilaginous embolic myelopathy in small animals. Vet Clin North Am Small Anim Pract. 40(5):859–69. © Elsevier. The ischemic injury caused by the arterial obstruction initiates a series of biochemical and metabolic events (similar to the secondary injury phenomenon described in Chapters 15 and 25), which result in neuronal and glial cell death. The gray matter, due to its greater metabolic demand, is generally affected more severely than the white matter. FCEM has been reported in dogs more commonly than in cats. At the time of writing, there are several studies including 24 or more dogs with FCEM (Table 9.1). The literature on feline FCEM is limited to case reports, and three case series for a total of 38 cats are reported in the literature (Table 9.2). Table 9.1 Summary of studies including ≥24 dogs of different breeds with a diagnosis of FCEM AD, antemortem diagnosis only; HD, histological diagnosis; NR, not reported. aAntemortem diagnosis of FCEM based on clinical presentation, myelography, ± CSF analysis. bAntemortem diagnosis of FCEM based on clinical presentation, ± myelography, ± CSF analysis (myelography was performed in 14 dogs, Dunie-Meringot 2007). cAntemortem diagnosis of FCEM based on clinical presentation, MRI, ± CSF analysis (1.5 tesla MRI [12]; 0.3 tesla MRI [13]). Table 9.2 Summary of case reports of cats diagnosed with FCEM NR, not reported. FCEM has been reported in large and giant breed dogs more commonly than in small- to medium-sized dogs [5, 8–13], and it is most common in nonchondrodystrophoid canine breeds. The reported male to female ratio in dogs ranges from 1:1 [8] to approximately 2.5:1 [9, 12]. The age at diagnosis in dogs ranges from 2 months [6] to 13 years and 5 months [13], with a median of 4–6 years in most studies [5, 8, 10, 12, 13]. Combining the data available on all the cats reported with FCEM in the literature, the domestic short hair is the most represented breed, the male to female ratio is 1.3:1, and the age at diagnosis ranges from 2 to 16 years (median, 10 years) [3, 14–22]. The typical clinical presentation is characterized by peracute (<6 h) onset of nonprogressive and nonpainful (after the first 24 h) and often lateralized neurological deficits consistent with the location, extent, and severity of the spinal cord infarction [12]. Rarely, neurologic dysfunction can progress for longer than 24 h, possibly as a result of additional embolizations or secondary spinal cord injury. In up to 80% of dogs diagnosed with FCEM, the dog was performing some type of physical activity, such as running, jumping, or playing, at onset of neurologic signs [8]. Signs of sudden and transient hyperalgesia (e.g., yelping as in pain) are observed by the owners at the onset of neurologic signs in up to 61.5% of affected dogs [8]. This transient hyperalgesia may be the result of stimulation of nociceptors in the endothelium of embolized spinal blood vessels [23]. This focal spinal hyperalgesia generally resolves in a few minutes to hours. Lateralization of neurologic signs has been reported in 52.8–86.5% of dogs with FCEM [5, 8, 10, 12, 13]. The asymmetry of signs can be very marked and often result in severely paretic or plegic limb/s on one side, while the contralateral limb/s has/have minimal deficits. This marked lateralization of signs is uncommon with other myelopathies and results from asymmetric branching of the intrinsic spinal cord vasculature, particularly the central branches of the ventral spinal artery, which have an asymmetric distribution in several spinal cord segments (Figure 9.1). The central arteries supply most of the gray matter and part of the lateral and ventral white matter of the spinal cord, sending branches to each side, or alternatively and irregularly to the left or right side of the spinal cord. The mean percentage of central arteries that send branches unilaterally is 79% in the cervical region, 58% in the thoracic region, and 37% in the lumbar region [24]. FCEM can also result in bilateral and symmetrical neurologic deficits. FCEM most commonly affects the L4–S3 and C6–T2 spinal cord segments in dogs with a histologic diagnosis [8, 10] and L4–S3 and T3–L3 in dogs with an antemortem diagnosis (Table 9.1) [5, 8, 10, 12, 13]. Neurologic deficits usually refer to the affected spinal cord segment (C1–C5, C6–T2, T3–L3, or L4–S3). However, in the acute stage of the disease, a decreased withdrawal reflex has been reported in severely paraparetic and paraplegic dogs with magnetic resonance imaging (MRI) changes consistent with FCEM within the T3–L3 spinal cord segment [12]. The majority of these dogs had an interruption of the cutaneous trunci reflex consistent with the site of FCEM on MRI [12]. It has been suggested that the transient decrease of the pelvic limb withdrawal reflex in dogs with acute thoracolumbar spinal cord injury is due to sudden interruption of descending supraspinal input on motor neurons and interneurons, fusimotor depression, and increased segmental inhibition [25]. This phenomenon is clinically relevant, as it may lead to an incorrect neuroanatomic localization, site of diagnostic investigation, and prognosis. FCEM most commonly affects the cervical spinal cord in cats. Lesion distribution in the 38 cats reported in the literature is C1–C5 in 11 cats, C6–T2 in 14 cats, T3–L3 in 7 cats, and L4–S3 in 7 cats. Lateralization of neurologic signs has been reported in 26 of 38 (68%) cats in which this information is available (Table 9.2). The main differential diagnoses for peracute, nonprogressive, lateralized myelopathy include ischemic myelopathy due to obstruction of the intrinsic spinal vasculature by material other than fibrocartilage, such as thrombi, or bacterial, parasitic, neoplastic, or fat emboli and acute noncompressive nucleus pulposus extrusion (ANNPE) (see Chapter 13). Underlying medical conditions that may predispose to embolization or thrombosis, including cardiomyopathy, endocarditis, hypothyroidism, hyperthyroidism, hyperadrenocorticism, chronic renal failure, and hypertension, should be considered and investigated, particularly in cats with peracute onset nonpainful and nonprogressive myelopathy. ANNPE occurs when nondegenerated nucleus pulposus extrudes during strenuous exercise or trauma, contuses the spinal cord, and dissipates within the epidural space, causing minimal to no spinal cord compression. The clinical presentation of dogs with ANNPE is often very similar to one of dogs with FCEM. Lateralization of neurological deficits has been reported in 62% of dogs with ANNPE [26]. The main clinical difference between ANNPE and FCEM is discomfort or hyperesthesia during palpation of the affected spinal segments, which has been reported in 57% of dogs with ANNPE, while it is uncommon in dogs with FCEM [12, 26]. High-field MRI and experience in neuroimaging also may help differentiate these two diseases [2, 12, 26]. Other differential diagnoses include compressive intervertebral disc extrusion, infectious and noninfectious meningomyelitis, neoplasia, hemorrhage (e.g., secondary to coagulopathy), and vertebral fracture, subluxation, or luxation. The history, onset, and progression of signs and the presence/absence of spinal hyperesthesia on clinical examination help to expand or narrow the list of differential diagnoses. Animals with FCEM may show discomfort on palpation of the affected spinal segment when examined within a few hours of onset of neurological dysfunction or anytime after onset of signs in case of concurrent spinal pathology such as spondylosis deformans. Alternatively, animals with spinal disorders commonly associated with some degree of spinal hyperesthesia (such as intervertebral disc extrusion) may show no obvious signs of hyperesthesia on palpation of the affected spinal segment because they are very stoic or due to recent administration of anti-inflammatory and/or analgesic medications. Pharmacological treatment may also interfere with progression of signs resulting in clinical stabilization or improvement of animals with disorders that would be progressive if untreated. Therefore, diagnostic investigations are needed to support the clinical suspicion of FCEM. The antemortem diagnosis of FCEM is based on history, clinical findings, exclusion of other etiologies of myelopathy (through diagnostic imaging and cerebrospinal fluid (CSF) analysis), and visualization of a lesion compatible with spinal cord ischemia on MRI. The definitive diagnosis of FCEM can only be reached through histological examination of the affected spinal cord segments. Plain radiographs of the spine help to exclude vertebral fracture, subluxation/luxation, neoplasia, and osteomyelitis/discospondylitis. Myelography helps exclude causes of myelopathy resulting in spinal cord compression, such as intervertebral disc extrusion. In dogs and cats with FCEM, myelography can be normal or may show an intramedullary pattern consistent with focal spinal cord swelling in the acute stage of the disease. An intramedullary myelographic pattern has been reported in 39–47% of dogs with histologically confirmed FCEM [8, 10]; however, this pattern can occur also with other myelopathies, including focal myelitis, intramedullary neoplasia, intraparenchymal hemorrhage, and ANNPE. A narrowed intervertebral disc space (on good-quality radiographs with optimal patient positioning) underlying the area of intramedullary myelographic pattern should prompt the suspicion of ANNPE. As with myelography, computed tomography (CT) and particularly CT myelography help rule out other causes of myelopathy, particularly those resulting in spinal cord compression. An intramedullary pattern of spinal cord swelling may be detected in the acute stage of FCEM; however, this pattern can occur also with other types of myelopathies. MRI of the affected spinal segments is the diagnostic imaging modality of choice to achieve an antemortem diagnosis of FCEM. In addition to excluding other causes of myelopathy, it allows visualization of signal intensity changes suggestive of spinal cord infarction. The MRI features suggestive of FCEM include a focal, relatively sharply demarcated intramedullary and often lateralized lesion (edematous infarcted tissue), predominantly involving the gray matter that is hyperintense to normal gray matter on T2-weighted fast spin echo (FSE) and FLAIR images and iso- or hypointense to normal gray matter on T1-weighted FSE images (Figure 9.2). Postcontrast T1-weighted FSE images may show mild and heterogeneous enhancement of the affected area, generally on the fifth to seventh day of disease [2, 12, 19, 27]. The lesion is often present in spinal cord segments near an intervertebral disc in which the nucleus pulposus has undergone degenerative changes resulting in a loss of signal intensity on T2W images. Figure 9.2 Sagittal (A) and transverse (B) T2-weighted FSE MRI of a 5-year-old border collie cross with peracute onset right-sided hemiparesis. Note the intramedullary hyperintensity dorsal to the C6 vertebral body (A) and (B) and C5–C6 intervertebral disc (A), affecting mainly the gray matter on the right side (B). There are no abnormalities within the epidural space (B). The intervertebral discs C4–C4, C6–C7, and C7–T1 show degenerative changes. MRI performed 24–72 h after onset of neurologic signs may reveal no intraparenchymal signal intensity changes in dogs with FCEM [12]. Dogs that are ambulatory in the acute stage of the disease are more likely to have a normal MRI than nonambulatory dogs [12]. The extent of the lesion on both sagittal and transverse T2-weighted MR images has been reported to be associated with the severity of clinical signs on presentation and with outcome [12, 28]. The degree and extent of the ischemic injury and the availability of high contrast resolution MRI influence the ability to detect signal intensity changes in the early stage of FCEM [12, 29]. Diffusion-weighted imaging can reveal abnormalities within a few hours of spinal cord ischemia, before the appearance of T2-weighted signal changes, and can help differentiating cytotoxic ischemic edema from the vasogenic edema associated with inflammatory lesions [30]. MRI can help to differentiate between FCEM and ANNPE. The MRI findings suggestive of ANNPE include a focal intramedullary hyperintensity overlying an intervertebral disc, with reduced volume and signal intensity of the nucleus pulposus on T2-weighted FSE images; narrowed intervertebral disc space; and presence of extraneous material or signal change within the epidural space dorsal to the affected disc, with absent or minimal spinal cord compression (Figure 9.3) [26, 31]. Figure 9.3 Sagittal (A) and transverse (B) T2-weighted MRI of a 9-year-old Wheaten terrier with peracute onset nonambulatory tetraparesis. Note the intramedullary hyperintensity above the C3–C4 intervertebral disc (A) and (B), the reduction in volume and signal intensity of the C3–C4 intervertebral disc nucleus pulposus (A), the narrowed C3–C4 intervertebral disc space (A), and the presence of extraneous material within the epidural space (mainly on the right side) dorsal to the affected disc (B). This MRI appearance is more consistent with ANNPE than FCEM. CSF analysis may be normal or may reveal nonspecific abnormalities including xanthochromia, elevated protein concentration, and mild to moderate neutrophilic or mixed pleocytosis. Polymerase chain reaction for different infectious agents on CSF may help to rule out specific causes of meningomyelitis. The definitive diagnosis of FCEM can be reached only by histological examination of the affected spinal cord segments. This reveals fibrocartilaginous material in spinal arteries or veins within or near an area of focal myelomalacia (Figure 9.4). The margins of the lesion tend to be well delineated from normal tissue. The lesion distribution reflects the territory of the embolized vessels and is therefore often asymmetric. The gray matter is generally more severely affected than the white matter. The lesion is initially ischemic but sometimes may become hemorrhagic. Figure 9.4 Gross (A) and histologic (B) sections of a spinal cord depicting the typical changes seen after fibrocartilagenous embolization and infarction. (Courtesy of Dr. Alexander de Lahunta.)
What is Fibrocartilaginous Embolism and Is It Related to IVDD?
Overview
Pathophysiology
Clinical presentation
Reference
Total Number of Dogs
Number of Dogs with AD and HD
Percentage of Large or Giant Breed Dogs
Age (Years, Median, and Range)
Male to Female Ratio
Percentage of Dogs with each Neuroanatomic Localization and/or Site of the Lesion based on Histology or MRI
Percentage of Dogs with Lateralization of Neurologic Signs
Percentage of Dogs with Nociception
Percentage of Dogs with Partial or Complete Recovery
C1–C5
C6–T2
T3–L3
L4–S3
[5]
24
ADa 19
79
5 (0.5–10)
1.2:1
16.7
20.8
33.3
29.2
70.8
NR
58.3
HD 5
[8]
72
ADa 26
54
5 (1–10)
1:1
3.8
3.8
42.3
50.0
80.8
88.0
73.9
HD 36
75
4 (0.3–7)
1.3:1
2.8
30.6
19.4
47.2
52.8
50.0
[10]
75
ADa 54
74
6 (1–11)
1.7:1
5.6
11.1
37.0
43.3
64.8
90.7
65.3
HD 21
81
5 (5–9)
1.6:1
9.5
33.4
14.3
42.8
55.0
33.3
[23]
26
ADb 26
54
4 (0.5–10)
1.4:1
15.4
19.2
34.6
30.8
73.1
96.2
66.7
[12]
52
ADc 50
81
6 (0.4–11.9)
2.5:1
0.0
28.9
26.9
44.2
86.5
94.2
84.0
HD 2
[13]
26
ADc 26
16
5.6 (0.4–13.4)
1.3:1
19.2
23.0
50.0
7.8
61.5
92.3
80.8
Case Report/Series
Number of Cats
Breed
Sex
Age (Years)
Duration of Progression of Clinical Signs
Neuroanatomic Localization
Neurological Dysfunction
Diagnostic Imaging
Follow-up
Histologic Diagnosis
Site of the Lesion based on Histology or MRI
[22]
1
DSH
FS
10
<24 h
L4–S3 right sided
Paraparesis, urinary, and fecal incontinence
Survey radiographs
Euthanized 6 days after presentation due to lack of improvement
Yes
L6–S3
[14]
1
NR
NR
12
NR
L4–S3
Paraplegia
NR
Euthanized
Yes
Lumbar intumescence
[15]
1
DSH
ME
12
48 h
C6–T2 left sided
Tetraparesis
Survey radiographs
Euthanized 2 days after presentation due to lack of improvement
Yes
Cervical intumescence
[16]
1
DSH
MN
12
<24 h
C6–T2 left sided
Hemiparesis
Myelography
Nearly complete recovery 7 weeks after diagnosis
No
Caudal cervical spinal cord
[17]
1
DSH
FS
9
<24 hours
L4–S3 left sided
Paraplegia, urinary incontinence, loss of nociception in the left pelvic limb
Myelography
Euthanized 12 days after diagnosis due to incomplete improvement
Yes
L4–S3
[18]
1
DSH
MN
8
5 days
C6–T2 left sided
Nonambulatory tetraparesis
Survey radiographs
Euthanized 7 days after presentation due to lack of improvement
Yes
C6–C7
[19]
2
Persian
MN
10
<24 h
C1–C5 left sided
Plegia of the left thoracic and both pelvic limbs, paresis of the right thoracic limb
MRI
Significant improvement 6 weeks after presentation. No recurrence in 4 years of follow-up available
No
C1–C3 left sided
DSH
FS
4
A few hours
C6–T2 left sided
Left-sided hemiplegia, mild right-sided hemiparesis
MRI
Full neurological recovery 2 weeks after presentation
No
C7 left sided
[20]
5
DSH
FS
10
24 h
T3–L3 left sided
Nonambulatory paraparesis
Myelography
Euthanized 1 day after diagnosis
Yes
L1
DSH
FS
11
36 h
C1–C5 left sided
Nonambulatory tetraparesis
None
Euthanized 3 days after diagnosis
Yes
C4
Maine coon
MN
7
<5 h
C6–T2 left sided
Tetraparesis
MRI
Euthanized 7 days after diagnosis due to lack of improvement
Yes
C6–C7
DSH
MN
10
2 h
C6–T2 right sided
Nonambulatory tetraparesis
MRI
Ambulatory 14 days after diagnosis
No
C5–C6
DSH
MN
7
<5 h
T3–L3
Paraplegia
MRI
Ambulatory 2 days after diagnosis
No
L2–L3–L4
[21]
6
Persian
FS
16
NR
C1–C5
Tetraparesis
MRI
Ambulatory 10 days after diagnosis
No
C2–C3
DSH
FS
13
NR
C6–T2 left sided
Paraparesis
MRI
Ambulatory 13 days after diagnosis
No
C7–T1 left sided
DSH
FS
8
NR
L4–S1 left sided
Paraparesis
MRI
Ambulatory 27 days after diagnosis
No
L5–L6 left sided
DSH
MN
13
NR
C1–C5
Tetraparesis
MRI
Ambulatory 7 days after diagnosis
No
C3–C4
DSH
MN
12
NR
C6–T2 left sided
Tetraparesis
MRI
Ambulatory 21 days after diagnosis
No
C7–T1 left sided
DSH
ME
2
NR
C1–C5 right sided
Tetraparesis
MRI
Ambulatory 10 days after diagnosis
No
C1–C2 right sided
[33]
19
14 DSH, 2 DLH, 2 Persian, 1 British SH
11 MN, 7 FS, 1 F
Median 10
<24 h
In all but 1 cat with 2 lesions on MRI, clinical and MRI neurolocalizations corresponded
Median and mean neurologic score of 3 (nonambulatory paresis)
MRI
Median time to recovery of ambulation was 3.5 days (3–19 days)
15/19 (79%) cats had a favorable outcome with a medial follow-up of 3 years and 1 month
Yes (1 cat)
C1–C5 (6 cats), C6–T2 (6 cats), T3–L3 (5 cats), L4–S1 (3 cats) with 1 cat affected by 2 separate lesions
Differential diagnoses
Diagnosis
Diagnostic investigations
Plain radiographs
Myelography
Computed tomography and CT myelography
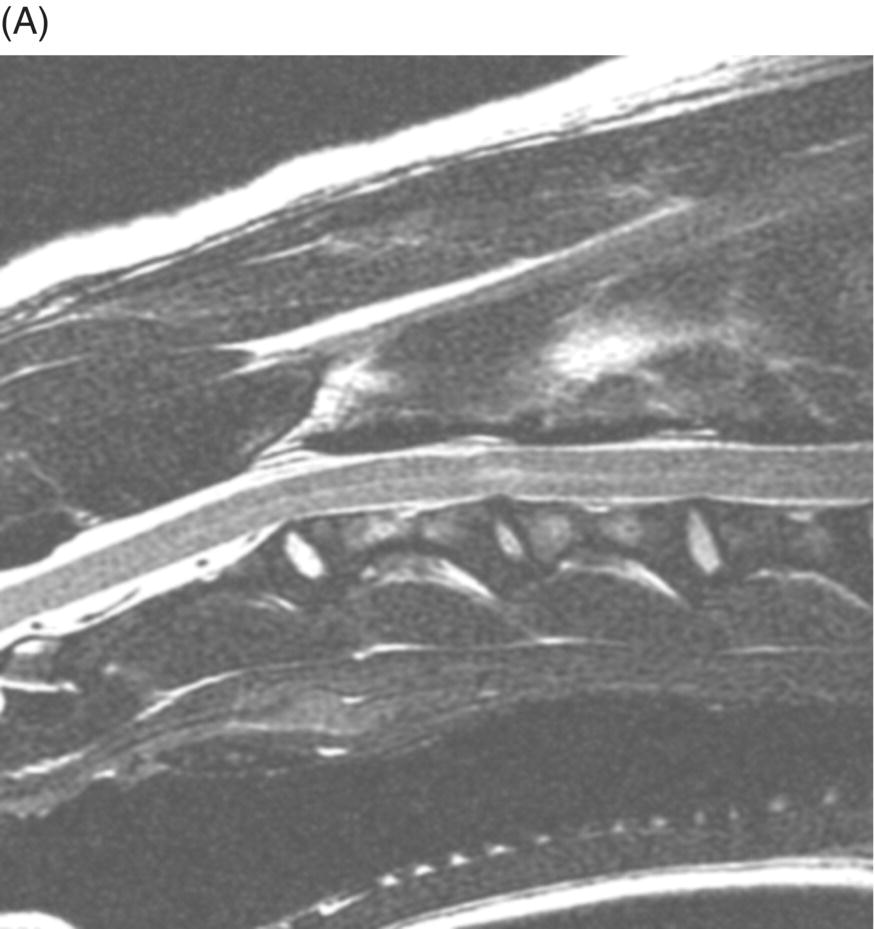
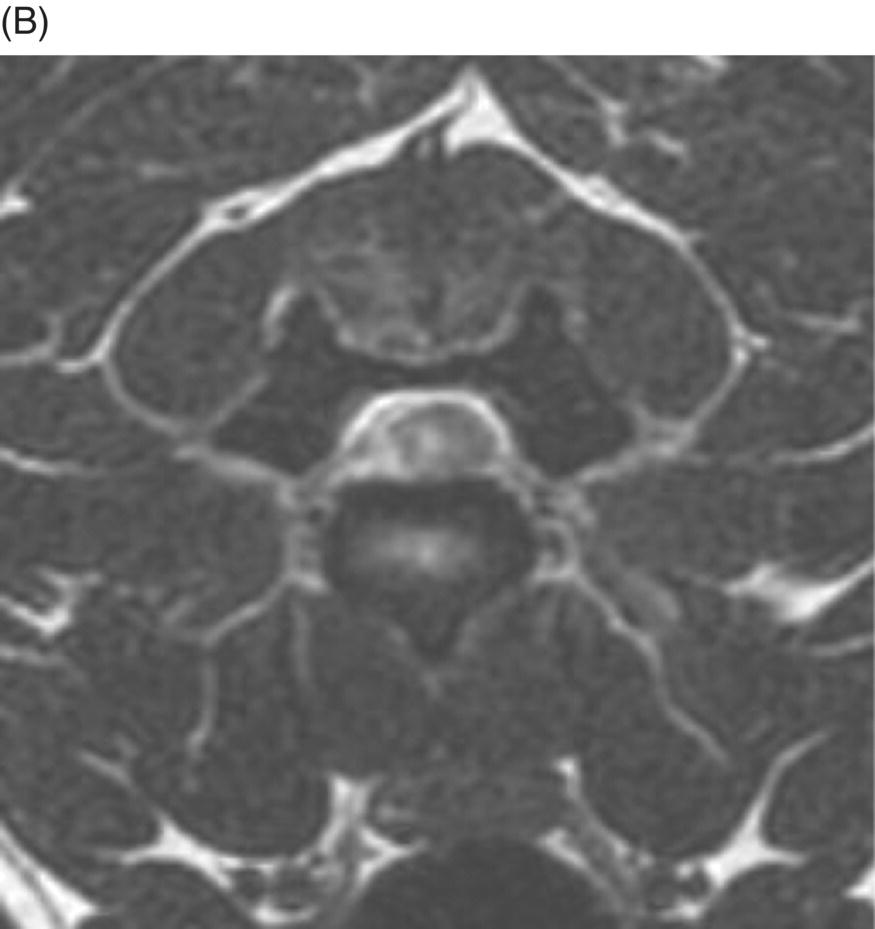
MRI
CSF analysis
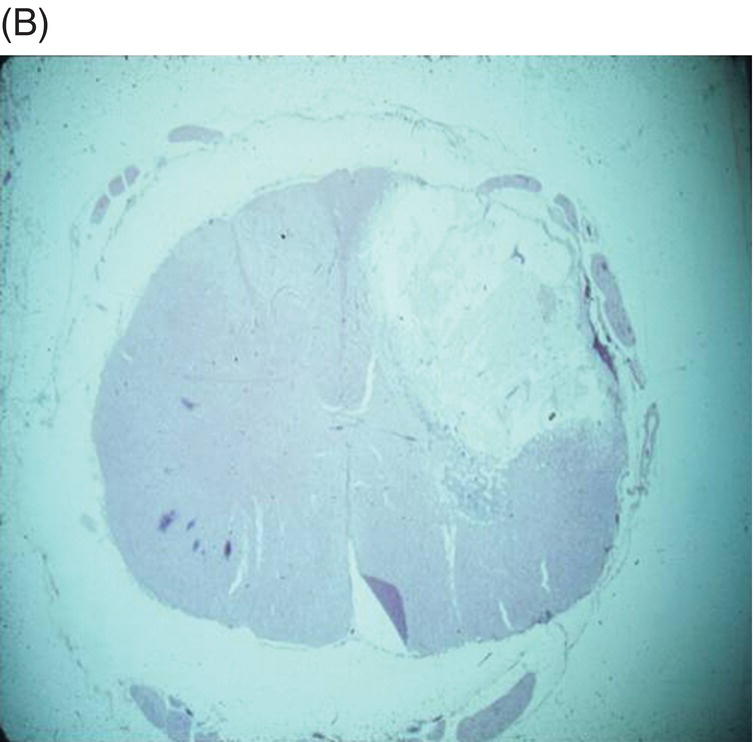
Histological examination

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


