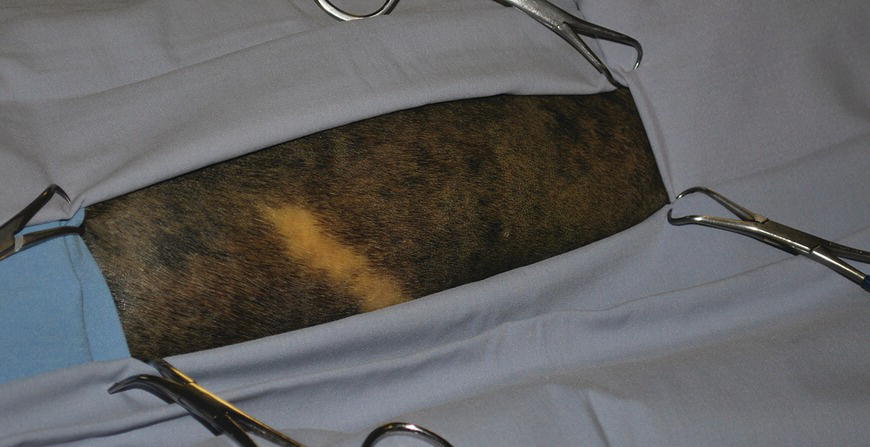
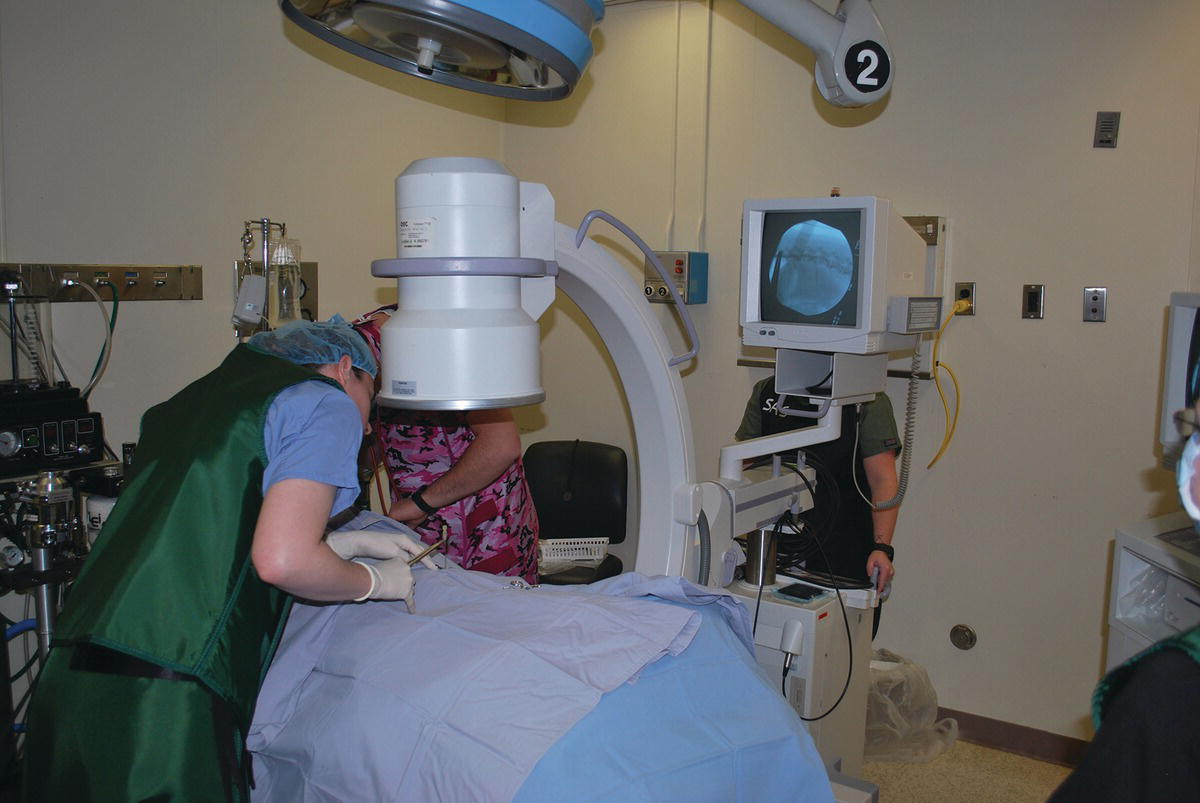
37 Kenneth Bartels Lasers for neurosurgery include both high-power surgical lasers for incisional/ablative procedures (i.e., carbon dioxide (CO2 − λ = 10.6 μm)), neodymium–yttrium aluminum garnet (Nd:YAG – λ = 1.064 μm), diode (λ = .805/.980 μm), holmium–yttrium aluminum garnet (Ho:YAG – λ = 2.1 μm), as well as low-level lasers for rehabilitative therapy. Carbon dioxide lasers, used for soft tissue surgical approaches to the spine, have the advantages of decreasing hemorrhage by photocoagulating small blood vessels and decreasing pain and inflammation by photothermal coagulation/sealing of nerves and lymphatics. Concurrently, when used correctly considering wavelength and laser–tissue interaction, the CO2 laser can potentially provide exquisite control for dissection and photoablation of tissue. Control of laser energy has also enabled the application of photothermal tissue welding using various organic solders that enhance weld strength of both blood vessels and peripheral nerves [1]. Fiber-delivered high-power lasers, including the Nd:YAG, diode, and Ho:YAG lasers, are used for tissue ablation/photocoagulation as well as for endoscopic or stereotactic applications [1]. Minimally invasive procedures include percutaneous laser ablation of the nucleus pulposus of intervertebral discs [2, 3]. Photodynamic therapy (PDT) utilizes laser energy of varying low-level power and wavelengths (visible to near infrared), as well as broader-band light sources such as light-emitting diodes (LEDs), to initiate a photochemotherapeutic action. A photosensitizer, such as a porphyrin compound, is coupled with the light source and specifically targets pathologic tissue using systemic or intralesional injections to release singlet oxygen as a toxic substance, especially for intracranial tumors in human patients. This chapter will concentrate on the use of the Ho:YAG for intervertebral disc ablation and attempt to outline efficacy as well as results of the current protocol. There will also be a brief discussion regarding the use of low-level lasers for rehabilitation of the neurologic patient. Appropriate and accurate neurologic evaluation coupled with proper treatment, both medical and surgical, for intervertebral disc herniation has been well described [4]. The use of disc fenestration as a definitive therapeutic procedure for disc displacement cannot be recommended [5–7] (see Chapters 18 and 35). Most veterinarians recognize that fenestration deserves a very restricted role in neurosurgical management of dogs with acute neurological signs. Some surgeons are reluctant to perform prophylactic fenestration, either simultaneously with decompression or at a later date as a separate surgical procedure, since a prolonged or additional surgical procedure presents opportunity for potential postoperative complications [8] (see Chapter 36). Another question that must be answered is the effectiveness of fenestration in preventing recurrence of disc displacement. In evaluating literature and establishing evidence-based conclusions, it is apparent that efficacy will continue to be questioned until objective effectiveness is established involving prospective evaluation to determine whether fenestration is truly successful in preventing future disc displacement [9] (see Chapters 35 and 36). Although prophylactic fenestration was reported to be successful in preventing future disc extrusions at fenestrated sites, the presumption that there could be an increase in disc displacement at adjacent, nonfenestrated sites was also considered possible. It was also concluded that multiple-site disc fenestration decreased the rate of recurrent intervertebral disc disease (IVDD) in small-breed dogs when compared to use of single-site fenestration [9]. With increased emphasis toward minimally invasive techniques that may reduce costs, decrease operative time, and improve patient care, minimally invasive prophylactic percutaneous laser discectomy at multiple disc sites is an alternative to surgical fenestration. The procedure has the potential for reduced complications associated with open surgery and to reduce recurrence for future disc displacement in the thoracolumbar (TL) area (T10–T11 to L4–L5) [2, 3]. A percutaneous approach for photothermal ablation or vaporization of the nucleus pulposus in lumbar discs by use of laser energy has been reported as a treatment of IVDD in human patients since 1975 [10–13]. The U.S. Food and Drug Administration has approved the Nd:YAG (λ = 1064 μm), frequency-doubled Nd:YAG (KTP/potassium titanyl phosphate – λ = 0.532 μm), and Ho:YAG (λ = 2.1 μm) lasers for use in intervertebral disc surgery [10–13]. Erbium–yttrium aluminum garnet (Er:YAG – λ = 2.8 μm) and carbon dioxide lasers (λ = 10.6 μm) have also been used experimentally, but with difficulty since transmission of the respective wavelengths through optical fibers is not possible or extremely restricted because of technologic limitations. Excimer laser (λ = 0.308 μm) has also been evaluated experimentally for use in laser discectomy [14]. The diode laser ((λ = 805 nm) coupled with indocyanine green (ICG) as a wavelength-specific chromophore has also been advocated as a method for percutaneous laser disc ablation (PLDA) in the canine model [15]. The Ho:YAG laser has advantages over other approved lasers [16–18]. The Ho:YAG wavelength is strongly absorbed by water, so depth of tissue penetration is limited, and zones of necrosis and collateral thermal effects are minimized because of the high water content of the nucleus pulposus. The Ho:YAG laser is also a pulsed laser (5–12 Hz), which allows cooling of tissue between pulses, potentially limiting tissue damage through a thermorelaxation phenomenon [19]. However, since the Ho:YAG laser operated at ambient room temperature is a pulsed laser, energy delivery elicits a photoacoustic or photomechanical effect which is problematic in potentially forcing additional disc material dorsally through the annulus fibrosus into the vertebral canal [20]. Finally, due to the solid state construction, portability, ruggedness, and ease of operation of most Ho:YAG laser systems, they become attractive for clinical application. As mentioned previously, studies using laser for discectomy in human patients focused on minimally invasive techniques that avoid damage to surrounding structures but still achieved ablation of the nucleus pulposus. Proponents of laser discectomy claim positive results in human patients that are related to a decrease in intradiscal pressure caused by a decrease in volume of the nucleus pulposus after ablation [21]. Further disc extrusion can also be prevented, although disc ablation is not effective for sequestered disc fragments [22]. Using the dog as a model, investigators have reported that acute and chronic histopathologic effects of PLDA on neighboring tissue are minimal [23]. In a more recent study, depending on the total energy applied during disc ablation, extensive photothermal damage can occur to cells of the nucleus pulposus which would be irreversible, but with lower energy/fluency levels based on in vitro studies, proteoglycan matrix synthesis may be promoted sufficiently to activate self-repair ability of the tissues [24]. Moreover, to improve accuracy and safety of needle placement, endoscopy, fluoroscopy, computerized tomography, and magnetic resonance imaging have been used to assess the precise location of laser fibers during percutaneous placement, which potentially minimizes intraoperative trauma [25, 26]. PLDA was initiated at Oklahoma State in 1992, using the canine model for determining potential adverse effects of the procedure when applied to human patients. Successful completion of the preliminary research project illustrated that percutaneous placement was possible in the TL area via a left dorsolateral approach in the dog [2, 3, 27]. Initially, a uniplanar fluoroscopic interventional radiology technique was employed. Later, a portable C-arm unit was used for percutaneous needle placement, which allowed the procedure to be performed aseptically in a surgery suite. This modification also allowed PLDA concurrently with a decompressive surgical procedure when indicated. Dogs are currently included in the Oklahoma State clinical protocol when they have a documented history of TL disc herniation and have recovered from either medical or surgical treatment. That is, they exhibit no neurologic abnormalities including lumbar pain, and ambulate normally. If a dog is presented with only lumbar pain and confirmed to have TL disc herniation through physical/neurologic and radiographic examination, usually confirmed with myelographic evaluation or computerized tomography (CT), it is treated conservatively for at least 2 weeks prior to laser disc ablation. If at any time the patient deteriorates neurologically, decompression and removal of the extruded disc are performed on an emergent basis after definitive localization of the lesion. Dogs included in the PLDA protocol are not administered oral or parenteral glucocorticoids for a minimum of 2 weeks prior to disc ablation. Acceptable candidates for PLDA undergo a presurgical evaluation that includes general physical and neurologic examinations, hematologic and serum biochemical analyses, and urinalysis. The dog is anesthetized and the hair on the left TL area is clipped from the caudal dorsocervical to the lumbosacral areas, and it is then aseptically prepared for surgery (Figure 37.1). Initially, the patient is placed in right lateral recumbency and fluoroscopic images from the portable C-arm are obtained. No repositioning of the dog is required when using the C-arm, which expedites the procedure in contrast to using the uniplanar technique on a radiology table (Figure 37.2). Lateral and dorsoventral views are obtained prior to and after needle insertion to ascertain potential anatomic variability of the spine and ascertain if there are any calcified intervertebral discs. Figure 37.1 Percutaneous laser disc ablation myelographic needle insertion site (dorsolateral aspect) of left epaxial area aseptically prepared for minimally invasive approach. Figure 37.2 Surgery suite is prepared for use of C-arm fluoroscopic imaging of percutaneous placement of myelographic needles. Using sterile technique, eight needles (20 gauge, 2.5 inch or 3.5 inch spinal needles) are placed percutaneously through the skin and epaxial musculature using a left dorsolateral approach. The spinal needle, with stylet in place, is carefully positioned through the annulus toward the nucleus of eight disc spaces (T10–T11 to L4–L5). Repeated 1–2 s, fluoroscopic images are taken to check position of the needle as it is advanced toward the disc. Palpation of the needle against bone and soft tissue is used to ascertain when the needle encounters the vertebral body or disc space. A characteristic soft, granular feel of the needle tip entering the annulus fibrosus is usually detected, and the needle is advanced carefully into the peripheral border of the nucleus pulposus. Entry of the needle into the nucleus often is accompanied by a slight extrusion of the needle stylet, attributable presumably to intradiscal pressure. Needles are placed sequentially into each disc from T10–T11 through L4–L5 (Figure 37.3). When needles are placed, the bevel of each tip is positioned, so it faces the center of the disc rather than the vertebral end plate. Insertion of spinal needles is often aided by use of a needle holder and digital manipulation to stabilize the needle near the hub. Both lateral and dorsoventral fluoroscopic images are examined and the image is recorded to ensure and document proper needle placement. That is, the tip of the needle enters the lateral aspect of the annulus fibrosus and penetrates no further than the approximate peripheral one-third of the nucleus pulposus. Considering that the laser fiber extends no further than 1–2 mm beyond the beveled needle tip, precise needle placement permits a more defined ablation site with less concern for potential collateral photothermal tissue damage. Figure 37.3 Eight 2.5 in. × 20 gauge myelographic needles have been inserted into intervertebral disc spaces T10–T11 to L4–L5 (8 total spaces). A reusable, sterile, cleaved 320 μm low-OH quartz optical fiber1
Use of Lasers in Veterinary Surgery and Percutaneous Laser Disc Ablation
Introduction
Percutaneous laser disc ablation
Technique for PLDA in dogs

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


