CHAPTER 36 Use of Interferon Omega for Skin Diseases
REVIEW OF COMPOUND
INTERFERONS IN GENERAL
Interferons (IFNs) are naturally produced glycoproteins first discovered in 1957. They are divided into two groups: type I, also called viral IFNs, and type II or immune IFNs. Type I IFNs include IFN-α and IFN-ω (also called IFN-alphaII1), both synthesized predominantly by leukocytes, and IFN-β, synthesized by most cell types but particularly by fibroblasts. All three bind to the IFN I-receptor. Type II IFN has only one member, the IFN-γ, which is synthesized mainly by T-lymphocytes and natural killer-cells (NK-cells) in response to antigenic or mitogenic stimuli.1 The goal of this chapter is to familiarize the reader with mechanistic and clinical uses of feline IFN omega (IFN-ω) in veterinary dermatology.
RECOMBINANT FELINE INTERFERON-OMEGA
Recombinant feline IFN-ω (rFeIFN) has a 170 amino acid (AA) sequence with a molecular weight of 25 kDa and is N-glycosylated. Its sequence shows a 60 per cent homology with human IFN-α and human IFN-ω, and a 35 per cent homology with human IFN-β. It belongs to the type I IFN group according to its specific AA sequence at the N-terminus, and its subclass is IFN-ω. The commercially available formulation (rFeIFN) is produced industrially by silk worm larvae (Bombyx mori) that have been infected with a recombinant baculovirus vector containing FeIFN cDNA.2
The purified rFeIFN-omega is acid-stable for more than 50 days at 4° C, and in vitro studies have shown that it has dose-dependent antiviral activities against feline herpesvirus, feline coronavirus, feline calicivirus (FCV), feline panleukopenia virus, and antitumor activities against a wide range of tumor cell lines of cats and dogs.1,3,4
PHARMACOKINETICS
Elimination after intravenous administration is biphasic and rapid. In the first or distribution phase, the half-life is 5 minutes. In the second or metabolic phase, the half-life is 31 minutes. rFeIFN is distributed mainly into kidneys and liver and is metabolized quickly in these two organs. The highest concentration of inactivated rFeIFN in urine can be measured within 15 minutes post-administration. rFeIFN does not cross the blood-brain barrier. The highest concentrations are found in kidney, liver, and thyroid tissue, and the lowest concentrations in muscle and adipose tissue.2
After subcutaneous administration of 5 million units (MU)/kg of rFeIFN, serum concentrations increase gradually and a maximum concentration (Cmax) of 577 U/mL occurs at 1.47 hours postadministration. Thereafter concentrations decrease slowly and are nondetectable after 24 hours. The elimination half-life is 1.72 hours.5 Even though serum levels are very short-lived, rFeIFN induces the activity of 2′-5′ oligoadenylate synthetase (OAS) in leukocytes in the peripheral blood and keeps the level elevated for 3 days.6 At least four isoenzymes of OAS are described in human beings. They are activated by double-stranded ribonucleic acid (RNA) and catalyze the formation of adenosine monophosphate (AMP) oligomers linked by a 2′-5′ diester bond. These oligomers then activate an RNAse, which is responsible for degradation of RNA at specific sequences, thus providing a system for the control of virus replication and gene expression.7
ADVERSE effects AND TOXICITY
Potential transient adverse effects postadministration include mild apathy, decreased appetite, sinus tachycardia, mild transient leukopenia, thrombocytopenia, and anemia. To date there are no studies on the long-term effects of rFeIFN. Its safety has not been determined in kittens less than 9 weeks of age, puppies younger than 1 month of age, and pregnant animals.5
Intravenous administration of 20 MU/kg rFeIFN to cats provoked a sinus tachycardia, mild apathy, mild increase of respiratory rate and body temperature; the latter two parameters were still within normal range.8
MECHANISM OF ACTION
Type I IFNs have multiple antiviral, antiproliferative, and immunomodulatory activities and exhibit autocrine as well as paracrine activities (Box 36-1).1 The IFN response is very fast and limits virus spreading, thereby buying time for the generation of an acquired immune response to the invading virus. IFNs exert their actions through cell surface receptors.
Box 36-1 The Activities of IFN Type I
ANTIVIRAL RESPONSE
Double-Stranded RNA-Dependent Protein Kinase R (PKR)
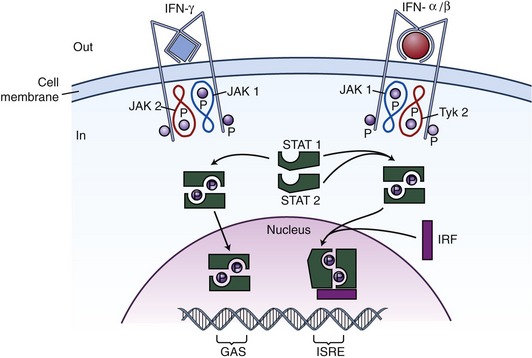
The IFN I receptor is composed of two major subunits, IFNAR1 (IFN-a receptor 1), associated with tyrosine kinase Tyk2, and IFNAR2 associated with Jak1. IFNAR 1 and 2 associate when IFN I binding occurs, thereby facilitating the transphosphorylation and activation of the two kinases, and creating a new docking site for STAT2 that is then phosphorylated and recruits itself STAT1, which also becomes phosphorylated. The phosphorylated STAT1/STAT2 heterodimers dissociate from the receptor and translocate to the nucleus where they associate with deoxyribonucleic (DNA)-binding protein p48 to form a heterotrimeric complex, ISGF3 (IFN-stimulated gene factor 3), which binds the ISRE (IFN-stimulated response element) of IFN I–responsive genes (Figure 36-1).
The primary step usually is inhibition of viral replication, induction of the protein kinase PKR, 2′,3′-oligoadenylate synthetase, and RNase L. All three of these induce apoptosis; RNA-specific adenosine deaminase ADAR1 and protein MxGTPase induce iNOS (inducible nitric oxide synthetase), repression of the cell cycle by the p202 IFN-I inducible gene product, down-regulation of c-myc transcription, and increase of MHC-I and -II molecules, all of which play an important role in immune responses to infections.7
Mainly by influencing Th1 type cytokine and chemokine induction and by regulating cytokine and chemokine receptor gene expression, IFN type I induces a Th1 type response and an adaptive CD8+ T-cell response that is likely to be efficacious against both chronic viral infections and neoplastic diseases that affect cats.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree