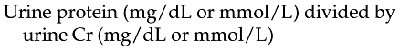CHAPTER 32 Urinary Tract Disorders
The Upper Urinary Tract
Diagnostic Methods
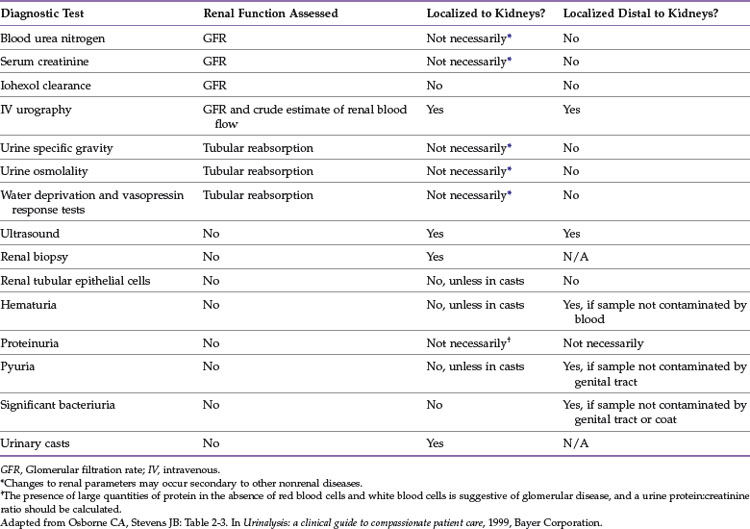
Various diagnostic methods are available for evaluation of the upper urinary tract, including imaging, renal function tests, urinalysis, urine culture, urine protein : creatinine ratio, and renal biopsy. Table 32-1 describes how diagnostic tests can be used to localize disorders.
Renal Size
Renal size is measured radiographically relative to the length of the second lumbar (L2) vertebra. Although there is no difference between the sexes in this parameter, there is an effect of gonadectomy on kidney size. A significant size difference was determined between intact and neutered cats, with intact cats having larger kidneys. Normal feline renal length ratios range from 1.9 to 2.6 for neutered cats and 2.1 to 3.2 for intact cats, which suggests that reproductive status should be taken into consideration when interpreting renal size.202 Box 32-1 lists the causes of renomegaly in the cat.
Renal Function Tests
Numerous tests have been evaluated for the assessment of glomerular filtration rate (GFR) or renal function. The standard 24-hour Cr clearance test is unwieldy, and renal scintigraphy is not widely available.121 One group evaluated a single injection of either inulin or Cr in normal cats and then compared plasma inulin and Cr clearances.The results showed that inulin may be a better indicator of GFR than Cr.166 The same researchers subsequently assessed iohexol and found that plasma clearance of this marker not only is a sensitive test for the detection of diminished renal function before changes in either BUN or Cr but also can be performed noninvasively in conscious cats.167 Another single-injection inulin clearance study compared inulin and iohexol clearance and showed excellent correlation between the two methods in their ability to detect alterations in GFR. The investigators concluded that an “inulin excretion test” sampling blood 3 hours after the administration of 3000 mg/m2 body surface area can be used for the assessment of renal function in daily practice.100 Excretory urography is another method to determine GFR; one study compared iohexol with amidotrizoate and concluded that iohexol was safer and produced better-quality urograms.6
Renal hemodynamics (resistance and pulsatility index) of intrarenal arteries has been studied using pulsed-wave Doppler; quantitative scintigraphy (99mTc-MAG3) was used to study relative renal function and relative renal blood flow. Of clinical relevance is that significant differences were found between awake and isoflurane-anesthetized cats for all pulsed-wave Doppler and quantitative renal scintigraphic measurements.165 Recently, a group evaluated an enzyme-linked immunosorbent assay (ELISA) test for gadolinium diethylenetriamine pentaacetic acid as a means to determine GFR. This test did not offer a sufficiently accurate estimation of GFR in cats when compared with plasma clearance of iohexol and plasma concentrations of BUN and Cr.203 Box 32-2 lists additional tests for renal function.
Urinalysis
• Urinary tract infection (kidneys, ureters, bladder, urethra—the last only if urine sample is voided)
• Urolithiasis or crystalluria
• Neoplasia: occasionally exfoliation of neoplastic cells occurs from urinary tract neoplasia
In addition, a voided sample evaluates the urethra, prostate, and vagina.
As with any laboratory test, it is possible to generate invalid and misleading results. The usefulness of a urine specimen is significantly affected by the timing of collection and the way it is collected, handled, stored, and examined. Additionally, the veterinarian should note all the drugs that a patient is receiving because many therapeutic agents affect the results of urinalysis (Box 32-3).177
BOX 32-3 Effects of Drugs on Urine Sample
1 Parenteral and oral fluids and diuretics
3 Acidifying and alkalinizing agents (therapeutic, dietary, or added to urine sample)
4 Iodinated radiopaque contrast materials
Collection Technique
The most reliable method for collecting urine from cats is by cystocentesis. Cystocentesis samples reflect prerenal, renal, ureteral, and bladder health. Voided samples reflect the aforementioned, as well as the urethra, prostate, vagina, and perineal fur. Further, voided samples reflect where the cat has urinated (e.g., the litter box, consultation table, or floor). The yield of the sediment must also be interpreted in light of collection technique. The bladder contracts circumferentially; however, sediment depends on gravity. Thus, for a cystocentesis-collected sample, the sediment yield may be improved by gently shaking the bladder just before inserting the needle.178 Voided samples also do not reflect sediment proportionately because of sediment remaining in the bladder as it contracts; thus samples collected in this manner may underestimate the degree of inflammation, crystalluria, and so on.
Handling the Urine Specimen
In an ideal world urine should be kept at room temperature and evaluated within 30 minutes of collection. Storage time and temperature alter and affect pH, USG, and crystal formation.7 If it cannot be examined in this time frame, the following suggestions will help preserve the integrity of the specimen.
1 Refrigerate at 41° F (5° C) for 2 to 3 hours or possibly overnight. The sample should be warmed to room temperature before analysis for accuracy of USG and for glucose assessment. Do not freeze the sample.
2 Protect the sample from light. Bilirubin becomes undetectable within 1 hour of exposure to sunlight.
3 Casts and cellular material deteriorate in alkaline urine. Over time, more crystals will develop depending on the pH of the sample; therefore the pH should be determined promptly.
4 Although preservatives for urine are available, each type alters the characteristics of the sample; specific information has been published elsewhere.178
Urinalysis Interpretation177,190
Color: Clarity and color are affected by many things, which, in turn, affect the USG value perceived with an optical refractometer. Conversely, urine color should also be interpreted in light of the USG. The color of the sample may be important insofar as it can affect interpretation of the colorimetric dry chemistries (urine strips). Color comparisons are subjective and are affected by colored urine constituents. Color should be assessed by a trained professional, in a consistently well-lit area and using fresh urine (Figure 32-1). Urine color may provide valuable information, including the following:
• Urine that is colorless is very dilute.
• Normal urine color ranges from transparent to light yellow, medium yellow, and amber. Normally, highly concentrated urine is amber as a result of increased urochrome or urobilin. Urochrome levels are also high in states of fever or starvation. Nitrofurantoin and riboflavin (vitamin B2) will cause urine to appear deep yellow.
• Orange-yellow color reflects high concentration, or excessive urobilin, bilirubin, or fluorescein (e.g., when used to identify a cat that is urinating inappropriately).
• Red, pink, red-brown, red-orange, or orange color suggests hematuria, the presence of hemoglobin, myoglobin, porphyrin, or warfarin. In humans ingestion of rhubarb, beets, phenothiazines, and other substances may cause this discoloration.
• A brown tint suggests methemoglobin; melanin; and, in humans, several drugs, including bismuth, mercury, and sulfasalazine.
• Yellow- or green-brown discoloration signifies the presence of bile pigment.
• Brown to black discoloration is actually brown or red-brown if evaluated as a thinner layer.
• Milky white urine is a result of chyle, pus, or phosphate crystals.
The accepted method for determining USG in cats is by using a refractometer. This tool assesses refractive index (ratio of velocity of light in air to the velocity of light in a solution). The refractive index is affected by the type and quantity of solutes present. Although refractometers are calibrated to a reference temperature, they compensate to a certain degree. They should be stored at room temperature. Veterinary refractometers measure a wider range of specific gravity and are therefore best suited for cat urine that may have USG in excess of 1.080. Human refractometers read only to 1.050. Digital refractometers appear to correlate with optical refractometers and have the advantage of less subjectivity.22 Some reagent strips include a pad for USG. Because these are developed for human urine, the highest value they detect is 1.030, which is inadequate for feline urine. Urinometers, devices that float in urine to measure USG, are imprecise. Osmometers assess osmolality rather than specific gravity. Regardless of the method used, all factors that affect refraction still should be taken into consideration.
The normal USG for a cat depends on hydration status and age. By the time a kitten is 4 weeks of age, USG is 1.020 to 1.038; full concentrating ability (up to 1.080) is reached by 8 weeks of age. In a dehydrated cat normal renal function (specifically, concentrating ability), is suggested by USG of 1.040 or above, depending on the diet fed. In cats fed exclusively canned food, a “normal” USG may be 1.025 or greater, whereas in cats fed exclusively dry food, USG should be 1.035 to 1.040 or higher.52 In a well-hydrated cat, it may fall between 1.035 and 1.060. Specific gravity varies within the same individual throughout the day; therefore a single urine sample with a low USG is not reliable evidence of a decline in renal function.
When nephrons are no longer able to modify glomerular filtrate, a fixed USG of 1.008 to 1.012 develops. USG of 1.007 to 1.039 in a dehydrated cat with or without azotemia is highly suggestive of renal insufficiency (or renal failure, depending on the degree of azotemia once the patient is rehydrated).176 Hypoadrenocorticism and hyperaldosteronemia are less common causes of such a drop in urine concentration. There is a subgroup of cats with impaired renal function that paradoxically remain able to concentrate urine to greater than 1.045, such that renal azotemia precedes a decline in USG.177 Because these patients are uncommonly identified, veterinarians must rely on finding USG of 1.045 or greater in the face of azotemia as indicating a prerenal cause for the azotemia.
Stress affects urine pH. In one study it was reported that the urine pH of a cat increased by 1.4 U when the cat was transported from home to a veterinary clinic. The authors concluded that the most likely cause was anxiety-induced hyperventilation (excessive panting).43 Another study suggested the opposite—namely, that increasing activity of the sympathetic nerves and the adrenal glands will most likely lead to increased metabolism, including catabolic conversion of proteins, which in turn increases sulfuric acid production and lowers urinary pH. This effect can also be seen in the fasted, inappetent, or anorectic cat.60
Urine glucose monitoring for insulin dose titration in the diabetic cat should not be used because the relationship between serum glucose concentration and that in the urine is variable.177
An increase in urinary bilirubin is associated with increased destruction of red blood cells (hemolytic disease), hepatocellular disease preventing normal elimination of this product, or bile duct obstruction (cholestatic disease). Altered selective permeability of glomerular capillaries in glomerulonephropathy can also potentially cause bilirubinuria by changing the renal threshold of affected nephrons. Bilirubinuria may precede clinically recognizable icterus and even bilirubinemia. Unlike in dogs, bilirubinuria is not found in normal cats, even in highly concentrated urine samples, presumably because of a higher renal threshold for bilirubin in this species.177
Hematuria indicates blood loss into any part of the urinary tract. Identification of the site of bleeding is the next step. Idiopathic renal hematuria has been recognized in cats and dogs. It is not known whether it is due to a vascular bed abnormality or to an abnormality in podocyte attachment, as occurs in humans.220
The aspects of the glomeruli that determine whether protein leaves the glomerular capillaries are size, electrical charge, and hemodynamics. In general, proteins at or below 45,000 daltons with a positive charge are most likely to pass through. Albumin is 66,000 daltons and has a negative charge, which is why there are negligible amounts of albumin in the urine of a cat with normally functioning glomeruli despite high plasma concentrations (Table 32-2). Plasma hemoglobin is normally bound to haptoglobin, making it too large to cross the glomeruli. When this binding capacity is exceeded, as may occur in hemolysis, then unbound hemoglobin can enter urine.
TABLE 32-2 Examples of Proteins Found in Urine, Their Relative Size, and Implied Reason for Presence
| Protein | Approximate Molecular Weight (Daltons) | Implication When Found in Urine |
|---|---|---|
| Smaller proteins (e.g., beta2-microglobulin, muramidase) | 11,800-14,400 | Unknown |
| Myoglobin | 17,600 | Ischemic or traumatic injury to muscles (heat stroke, electrocution, severe muscular exertion, snake bite, crush injury) |
| Bence Jones proteins | 22,000-44,000 | Multiple myeloma |
| Alpha1-microglobulin | 27,000 | Unknown |
| Alpha1-acid glycoprotein | 40,000 | Unknown |
| Hemoglobin (unbound to haptoglobin) | 64,500 | Low urine specific gravity, alkaline urine, intravascular hemolysis |
| Albumin | 66,000 | Significant glomerular disease |
Adapted from Osborne C, Stevens J, Lulich J et al: A clinician’s analysis of urinalysis. In Osborne C, Finco D, editors: Canine and feline nephrology and urology, ed 1, Baltimore, 1995, Williams & Wilkins.
Interpretation of the significance of protein in urine depends on the USG. For example, mild 1+ proteinuria with USG of 1.010 implies greater protein loss than 1+ protein in a sample with USG of 1.040. Localization of the protein source requires knowledge of collection technique and the urine sediment constituents (Table 32-3).
TABLE 32-3 Localization, Causes, and Findings in Proteinuria
| Urinary Protein Source | Findings |
|---|---|
| Hemorrhage into urinary tract (trauma, inflammation, neoplasia) | Occult blood test +, TNTC red blood cells + white blood cells in sediment, high protein |
| Inflammation in urinary tract | Variable number of white blood cells in sediment, protein rarely >2+ unless concurrent hemorrhage |
| Infection in urinary tract | Many white blood cells and bacteria in sediment, protein rarely >2+ unless concurrent hemorrhage |
| Glomerular and/or tubular disease | No occult blood, no significant sediment findings, +/− casts, protein higher in glomerular than tubular disease |
| Functional extrarenal causes for transient glomerular changes (e.g., fever, stress, extreme environmental temperatures, seizures, venous congestion of kidneys, exercise) | No occult blood, lack of significant sediment findings, +/− casts, high protein, transient |
| Hemoglobinuria, myoglobinuria | Variable amounts protein, no significant sediment findings |
TNTC, Too numerous to count.
Adapted from: Osborne C, Stevens J, Lulich J et al: A clinician’s analysis of urinalysis. In Osborne C, Finco D editors: Canine and feline nephrology and urology, ed 1, Baltimore, 1995, Williams & Wilkins.
The reader is referred to the ACVIM Consensus Statement143 for a comprehensive discussion of causes, significance, identification, and management of proteinuria in dogs and cats. This topic is discussed in greater detail later in this chapter.
Storage of urine can alter crystalluria dramatically and therefore the clinician’s diagnosis and treatment planning. A study performed to look at the effects of storage on the diagnosis of crystalluria and casts in cats with no history of urinary tract disease was performed. Crystalluria was detected in at least one aliquot in 92% of stored samples as opposed to only 24% of samples examined fresh. Regardless of whether the sample was stored or fresh, urine from cats fed an exclusively canned diet did not have crystals.208
Urine Culture and Sensitivity
In cats with chronic kidney disease (CKD), the prevalence of urinary tract infections (UTIs) is 22%; in cats with uncontrolled DM and in cats with hyperthyroidism, it is 12%. Many cats with UTIs have no clinical signs of lower urinary tract disease or changes in their laboratory values indicative of infection.156 Because UTI is so common in cats with hyperthyroidism, DM, and CKD, urine culture and sensitivity is recommended as part of the minimum database for cats with these conditions, especially if the USG is 1.030 or lower regardless of sediment and, in more concentrated samples, if significant numbers of white blood cells or bacteria are seen. Box 32-4 shows causes of negative growth on urine culture in the face of the detection of bacteria in the sediment.
BOX 32-4 Factors that May Explain Lack of Growth on Urine Culture when Bacteria Were Identified in the Sediment
1 Bacteria nonviable in the urine at time of collection (e.g., antimicrobial therapy, immunologic defenses)
2 Urine sample improperly handled or preserved, causing death of bacteria
3 Organisms fastidious, did not survive time between collection and culture outside of the body
4 Improper culture technique (e.g., anaerobic organism processed as an aerobe)
Urine Protein : Creatinine Ratio
Microalbuminuria
In humans microalbuminuria is predictive of future renal health. In cats the predictive significance of microalbuminuria is not understood at present. The recommendation of the International Renal Interest Society (IRIS; http://www.iris-kidney.com/) is to continue to monitor this level of proteinuria.96
The reader is referred to the ACVIM Consensus Statement143 for a comprehensive discussion of the causes, significance, identification, and management of proteinuria in dogs and cats. (This topic is also discussed later in this chapter.)
Imaging of the Kidney and the Ureters
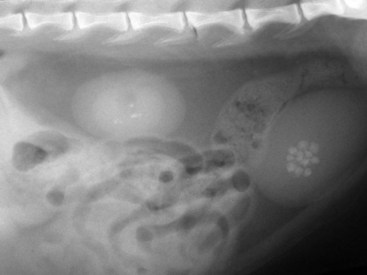
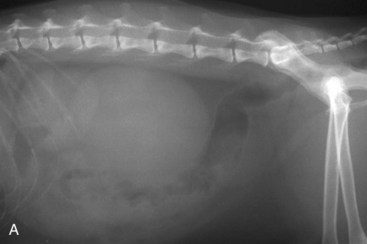
Imaging studies commonly used in the assessment of the upper urinary tract of cats include survey radiography, contrast excretory studies, and ultrasound. The normal signal intensities have been determined for magnetic resonance imaging (MRI) of the normal feline cranial abdomen.173 Each test has a role to play in patient diagnostics. Plain radiographs allow assessment of the number, size, location, and density of the kidneys. The limitations of survey radiography include an inability to visualize kidneys in the thin cat lacking retroperitoneal fat or if retroperitoneal fluid is present. Moreover, survey radiography cannot delineate problems of the renal pelvis or of the ureters unless there is radio-opaque material present (e.g., renoliths or ureteroliths, dystrophic mineralization) (Figure 32-2). Fecal material may obscure the outflow tract. Abdominal compression (e.g., using a wooden spoon over the organ of interest or a general abdominal wrap) may help enhance the image of a specific area by eliminating some of the superimposition encountered with survey radiographs.11
Excretory urography is the technique of choice when the renal parenchyma, renal pelvis, or ureters are of concern. This study may be useful to establish the relationship between a renal mass and the pelvis or ureter, to locate an avulsed or congenitally displaced kidney, to identify and possibly distinguish between acute and chronic pyelonephritis, to detect a nonfunctional kidney, to diagnose hydronephrosis, and to outline radiolucent uroliths.119
Ultrasound has the advantage of being noninvasive and quick. It is safer for the patient because no contrast material is needed and safer for the patient and owner because there is no exposure to ionizing radiation. It can be used to guide renal biopsy should this be indicated. This modality is useful for evaluating renal masses, cysts, diseases of the renal pelvis (calculi, hydronephrosis, pyonephrosis53) and adjacent abdominal structures. Although it is difficult to visualize the normal feline ureters ultrasonographically, various abnormalities associated with ureteral dilation may be revealed, including ectopic ureter, ureterocele, and certain causes of ureteral obstruction. Ultrasonographic evidence of hypoechoic subcapsular thickening in feline kidneys is associated with renal lymphosarcoma.215 Ultrasound guidance facilitates certain interventional diagnostic procedures for the ureters.135 Its limitations include the inability to visualize structures through abdominal air or bone (i.e., pelvic structures occlude the urethra).
Renal Biopsy
Histopathology of a kidney biopsy sample can provide information by revealing a pathologic process other than chronic interstitial nephritis. Patient selection is important. This procedure should be recommended in those individuals whose treatment will change because of information the results provide. Thus those with proteinuria believed to be of glomerular origin, those with ultrasound evidence of infiltrative disease, or those in ARF are appropriate candidates. The benefits of biopsy in patients with renomegaly outweigh risks; in general, however, for patients with small, scarred kidneys, it is unlikely to be of use. To obtain the best possible results, the veterinarian must be well prepared and understand the laboratory’s sample-handling requirements.214 The laboratory should ideally be able to perform not just light microscopy but also electron microscopy (EM). The latter requires a specific transport medium, gluteraldehyde, which is available through the laboratory.
Two centers that provide this comprehensive service are the Texas Veterinary Renal Pathology Service (Texas A&M University, College Station, Tex) and the Veterinary Pathology Diagnostic Centre (Utrecht University, Utrecht, The Netherlands). They encourage submission as part of the World Small Animal Veterinary Association (WSAVA) Renal Standardization study in order to increase understanding of glomerular diseases of dogs and cats. EM is needed for complete histologic examinations, especially to define early stages of kidney diseases (minimal changes disease, epithelial tubular pathologies, and tubular basement membrane and glomerular basement membrane changes). Along with clinical, histologic, histochemical, and immunologic examinations, it is an essential method for diagnosis and prognosis of renal disease.198 Because complications after renal biopsy are usually minor, provided the biopsy is performed properly, this tool for diagnostic evaluation should be encouraged.164,214
A sample may be collected by tissue-core biopsy (e.g., Tru-Cut) percutaneously with ultrasound guidance or surgically by laparotomy or keyhole approach. The first technique requires only very good sedation and local analgesia, whereas the latter requires general anesthesia. Regardless of technique, monitoring for postprocedural bleeding is critical. This and other possible complications (e.g., peritonitis, local infection, neoplastic seeding) still occur in fewer than 2% of patients undergoing percutaneous sampling. Unless the procedure inadvertently interferes with significant vasculature, GFR is not substantially affected.74 By guiding the biopsy needle through cortex only from pole to pole, the veterinarian is able to avoid the medulla and the medullary-cortical junction and significant vascular supply (Figure 32-3). The patient should be monitored to ensure that adequate analgesia is being used; palpation over and around the biopsy site will be a good guide. Renal biopsy is contraindicated in patients with coagulopathy, those receiving drugs affecting bleeding, and those with cavitated lesions (e.g., vascular lesion, cyst, abscess, hydronephrosis) to avoid leakage of contents or bleeding.26 More information on performing renal biopsy can be found later in this chapter.
Inherited, Congenital, and Developmental Renal Diseases
Renal anomalies are rare in cats. The most common familial disorders in cats include renal amyloidosis, renal dysplasia, polycystic kidney disease, glomerular basement membrane disorders, and tubular dysfunction (Fanconi’s syndrome).98 Errors of embryologic development that have been recognized in cats include agenesis, renal fusion, ectopic kidney, and cysts.
In reports of renal agenesis, the right kidney is more likely to be absent along with its ureter (in toto or partially). In affected females the uterine horn on the same side is also partially or completely missing. The ovary, having a different cellular origin, is present. In affected males the vas deferens and epididymis may be lacking, but the ipsilateral testicle will be normal.81
In the recent literature, two cases with urogenital abnormalities have been reported. The first, a 9-month-old female domestic shorthair, was lacking the right kidney and ureter and a segment of the uterine horn on the same side. The cat was brought in because of acute vomiting, depression, and shivering caused by hydrometra of the right uterine horn segment.51 The second case described was of a 1.5-year-old female Persian with azotemia and inappetence. Like the earlier case, the cat also had renal and ureteral agenesis with segmental aplasia of the uterine horn on the right side; however, in this cat the affected uterine segment was caudal, resulting in cranial uterine horn distention.91 In a study in which 257 Ragdoll cats were screened for polycystic kidney disease, 0.8% were identified with renal agenesis/aplasia.179 In a large study of more than 53,000 cats presented for ovariohysterectomy, uterine anomalies were detected in 0.09% of cats (n = 49). The kidneys were also evaluated in 34 of the affected cats, and ipsilateral renal agenesis was found in 29% (10 of 34).158
Crossed renal ectopia with fusion was identified in an adult neutered male cat that was presented for polyuria and polydipsia and shown to have renal disease and hypertension. Imaging revealed an ectopic left kidney fused with an orthotopic right kidney.8 Bilateral renal dysplasia was found in a 5-month-old Norwegian Forest Cat; histopathology revealed primary tubular disorganization and changes in the glomeruli.10
Membranoproliferative glomerulonephritis (GN) was reported in a 9-month-old domestic shorthair cat in Japan.15 In another report a series of 8 young, related Abyssinian cats of both sexes presented with hematuria and were found to have varying degrees of proteinuria. Six of the eight developed nephrotic syndrome with peripheral edema. Histopathology revealed mild glomerular changes, including mesangial hypercellularity with adhesions between Bowman’s capsule and the glomerular tuft consistent with focal proliferative glomerulopathy. Genetic analysis was not available in this report.222
In Norway, 11 Ragdoll cats were evaluated after two unrelated queens were found suddenly dead as a result of oxalate nephrosis with chronic or acute-on-chronic underlying renal disease.105 Renal abnormalities were found on ultrasound of five cats. Although investigated as an inherited condition, the etiology and mode of inheritance were not elucidated. Primary hyperoxaluria was ruled out by urine oxalate and liver enzyme analysis.
Cystic Diseases
Polycystic Kidney Disease
Polycystic kidney disease (PKD) is found in Persian, Himalayan, and Exotic Shorthair cats around the world and is reported extensively in the United States,58 United Kingdom,46 Australia,17,20 France,18 Italy,31 and Slovenia.69 The prevalence rates in these studies are between 40% and 50%. Many young Persian cats are asymptomatic, and renal function may not begin to decline until the cat is 7 or 8 years of age. Other breeds of cats manifest this condition rarely; a case report describing a Chartreux cat was recently published.217 It has been shown to have an autosomal dominant mode of inheritance in all of these breeds.27,153 All affected individuals are heterozygous for the causative mutation; homozygous individuals die in utero. Concurrent with the renal cysts, unilocular or multilocular cysts may be seen in the liver with or without congenital hepatic fibrosis,33 as well as other abdominal organs (Figure 32-4).69
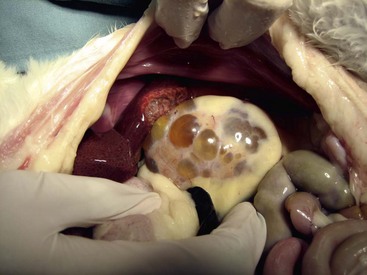
FIGURE 32-4 This necropsy image shows the typical appearance of a polycystic kidney in a Persian cat.
(Courtesy Dr. Susan Little.)
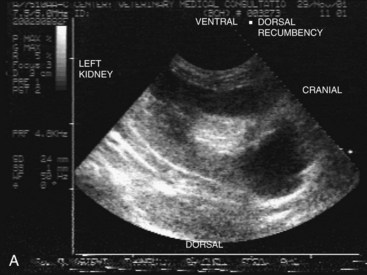
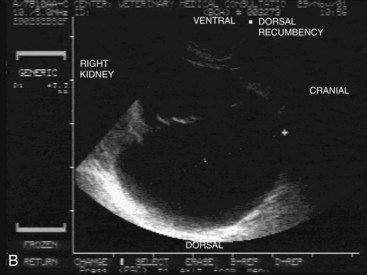
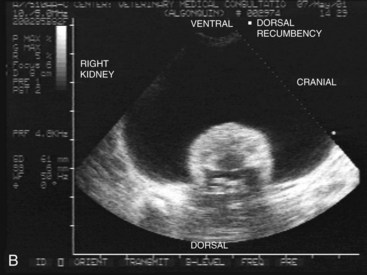
Cats may exhibit no clinical disease, slowly progressive renal insufficiency as adults, or significant disease as young cats, with marked renomegaly. Because of the variable clinical picture and because of the autosomal dominant inheritance in a pedigreed population, screening to identify affected individuals is essential. The condition may be unilateral or bilateral with irregular, enlarged kidney (or kidneys) on palpation. Radiographically, the affected kidney will be irregularly enlarged; on excretory urogram multiple radiolucent areas will be seen. Ultrasound is readily available and may help identify affected cats long before they show clinical signs. Thus sonographic screening is recommended in kittens older than 13 weeks of age; a skilled ultrasonographer can detect cysts in affected kittens as young as 6 to 8 weeks of age. Absence of cysts at a young age does not predict that the kitten will not develop them at a later age. Conversely, a cat with cysts may never show clinical disease. Cysts are located in the medulla or cortex (or both) and are anechoic to hypoechoic, round to irregularly shaped structures of variable size that may grow over time (Figure 32-5). Affected kidneys have indistinct corticomedullary junctions and may have mineralized foci. Further evaluation using intravenous contrast medium allows more definitive identification of cysts with computed tomography (CT), as well as identification of distortion of the renal pelves by cysts.189
Genetic testing has been developed to detect a C→A transversion at position 3284 on exon 29 of the PKD1 gene, resulting in a stop mutation. A real-time polymerase chain reaction (PCR) assay using fluorescent hybridization probes and melting curve analysis has recently (2009) been developed that may be as reliable but faster than previous methods.63 It is, however, recommended to use both ultrasound as well as genetic testing to improve sensitivity and specificity32 to decrease the prevalence of the disease in the Persian population.58 PKD is the leading cause of renal disease in Persian and Persian-related breeds.
Therapy for hepatic and renal cysts is warranted when there is significant compression of adjacent tissue or pain from capsular stretch. Drainage may be performed using ultrasound guidance. In one study of dogs and cats, the drained cysts were infused with alcohol for two periods of 3 minutes. Short-term discomfort was noted in all the patients, with anorexia, lethargy, or vomiting occurring in some.229
Interestingly, although dogs with renal cysts and humans with PKD have hypertension, cats with PKD are normotensive. One study that looked at the effects of the angiotensin-converting enzyme inhibitor (ACE-I) enalapril on BP, renal function, and the renin-angiotensin-aldosterone system (RAAS) in affected cats compared with healthy controls found that the ACE-I reduced BP in all and resulted in changes in RAAS enzyme activities and hormone concentrations with minimal effects on renal function.163
Perinephric Pseudocysts
Much less common than PKD, perinephric pseudocysts (PNPs) surround one or both kidneys, which may be normal or subnormal in size. Not a true cyst, the fibrous sac lacks an epithelial lining; thus the fluid it contains is extravasated rather than secreted. There is no single etiology, and this condition may follow renal trauma (urine leakage resulting in scarification) or perirenal fat necrosis (resulting in inflammation), may be associated with neoplasia (e.g., transitional cell carcinoma186), or may be categorized as idiopathic. Regardless of the initiating cause, anechoic fluid accumulates between the renal capsule and the parenchyma. The sac is often at a pole, but the fluid dissects between the kidney and its capsule or is extracapsular. Fluid cytology reveals a transudate with urea nitrogen close to that of serum. Because the structure does not communicate with renal parenchyma, contrast does not fill it, and on ultrasound it is seen to envelop the kidney rather than exist within it. One report describes a case in which the pseudocyst communicated with the pleural space, resulting in hydrothorax. Unilateral nephrectomy of the affected kidney resulted in resolution of the hydrothorax.191
Affected cats are generally older (older than 8 years of age); there is no sex or breed predisposition.21,175 The lesion is initially detected on palpation; renal insufficiency may be diagnosed on the basis of serum biochemistries and urinalysis, either due to associated interstitial fibrosis or the effect of compression. Imaging reveals the nature of the lesion (Figure 32-6).
Surgical resection of cyst walls is recommended, although reduction by drainage may provide temporary relief. There is one report of cyst wall omentalization with good long-term outcome.108 Another case report describes laparoscopic fenestration of the capsule wall with resulting improvement in GFR of the affected kidney; no relapse was seen.157 In other cases renal disease progresses, with the outcome being related to the severity of the renal disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree