Chapter 1 Urinalysis
Introduction
B. Urinalysis not only is helpful in evaluation of patients with urinary tract disease but also in those with systemic disease affecting many other body systems.
D. Abnormalities on urinalysis often occur before changes occur in serum biochemistry (i.e., urinalysis can be very sensitive).
E. Urine should be submitted for analysis at the same time blood is submitted for biochemical analysis.
Collection of Urine
A. Urine collection technique influences interpretation of the results. Urine specimens should be placed in containers that are clean and free of chemical contaminants (e.g. detergents, disinfectants, bleach). Ideally, collect 10 to 12 mL of urine for analysis. At a minimum, 3 mL should be submitted.
Manual Expression of Urine by Digital Palpation of the Bladder
Catheterization
Technique
a. Maintain sterile technique while using a catheter, gloves, and proper patient cleansing and disinfection. A gentle approach is necessary to minimize trauma to the urinary tract. The catheter should never be advanced by force, and a ruptured urethra or bladder can be a consequence of poor technique.
(1) Male dogs (Figure 1-1): Catheter types include polypropylene, polyvinyl, and balloon-tipped ureteral catheters for use in humans. Softer catheters are chosen for indwelling urinary catheterization. A catheter size of 3.5 to 10 French (1 French unit = 0.33 mm) is recommended, depending on the size of animal. The appropriate length of catheter to insert is chosen by estimating the length from the external urethral orifice to the bladder neck by over-laying the catheter on the animal’s body. The patient is positioned in lateral recumbency, excessive hair near the tip of the prepuce clipped, and the penis extruded by an assistant. The penis and external urethral orifice are gently cleansed and rinsed with a mild disinfectant (e.g., benzalkonium chloride) and sterile saline. The packaging containing the sterile catheter can be cut to facilitate manipulation of the catheter without contamination. Liberally coat the end of the catheter with sterile lubricating jelly and insert the catheter into the external urethral orifice. The catheter should be directed parallel to the abdominal wall to facilitate its passage. Resistance may be encountered in the perineal region and also at the level of the ischial arch. If necessary, external perineal palpation or rectal palpation can be used to redirect the catheter tip. To avoid trauma to the bladder, the catheter is passed only as far as needed to obtain urine drainage. In cases of obstruction the catheter may be advanced farther to allow better drainage of urine.
(2) Female dogs (Figure 1-2): Direct visualization of the urethral orifice using a speculum is preferred over a blind technique so as to avoid contamination of the urinary tract with genital bacterial flora. A variety of types and sizes of specula are available; those with self-contained light sources are preferable. Anuscopes designed for humans are easily adapted for use in most female dogs. Otoscopic and vaginal specula of various sizes also can be used. Catheters described above for use in male dogs can be used in females, as can Foley catheters and metal bitch catheters. Stylets are necessary for polyvinyl catheters and for small-caliber polypropylene catheters. The patient usually is placed in sternal recumbency, but different body positions may facilitate catheter passage in some circumstances. The external genitalia should be clipped of hair, cleansed, and disinfected. Pass the speculum or anuscope dorsally and then cranially, minimizing vaginal contamination. It is easier to pass the speculum beyond the urethral orifice and then retract it slowly until the orifice comes into view. Liberally coat the end of the catheter with sterile lubricating jelly and insert it into the external urethral orifice. The urinary catheter is advanced under direct visualization until urine is obtained.
(3) Male cats: Catheter types include medical grade Silastic, Jackson-style (Cook Veterinary Catheters), polyvinyl, and polypropylene catheters. Tom cat catheters are rigid polypropylene catheters available in 3.5 F as open-end or side-holes varieties. The open-end catheter is shorter than the side-hole catheter and consequently is not always reliable for draining the bladder. Polyvinyl catheters are available in 3.5 F or 5 F sizes. The cat is placed in lateral or dorsal recumbency with its hind limbs pulled caudally. The penis should be extended caudally to eliminate the natural curvature of the urethra. A perception of distal urethral obstruction may be created if the penis is not sufficiently extended. Coat the end of the catheter with sterile lubricating jelly and advance it into the urethra until urine is obtained.
(4) Female cats: A small otoscope can be used to visualize the urethral orifice. The vestibule of most female cats can accommodate a small anuscope speculum. Small female cats can be catheterized blindly. Rigid polypropylene or polyvinyl catheters generally are used. Rarely, small Foley catheters are used. Generally, a 6 to 8 F catheter can be accommodated. Female cats almost always require sedation to allow urinary catheterization. The cat is placed in ventral recumbency with its hind limbs over the edge of the table, and the tail extended dorsally. Some operators prefer dorsal recumbency. The catheter should be gently inserted and directed slightly dorsally along the ventral floor of the vestibule to enter the external urethral orifice. A small otoscope speculum can be used to visualize the external urethral orifice and facilitate catheterization passage.
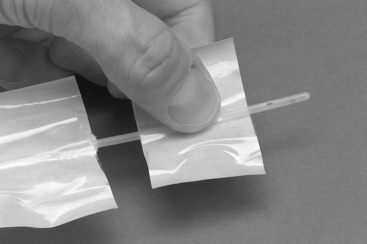
FIGURE 1-1 Sterile handling of a urinary catheter for a male dog. The sterile catheter package has been cut with scissors to facilitate passage of the catheter while at the same time maintaining sterile technique. This technique allows the catheter to be manipulated without the use of sterile gloves.
Disadvantages
a. The normal bacterial flora of the distal urethra can be introduced into the bladder during passage of the catheter.
d. Rupture of the urethra or bladder may occur if excessive force is used to pass the catheter or if the tissues are friable.
e. Never chosen for routine collection of urine from male cats due to the risk of urethral trauma and subsequent obstruction.
Cystocentesis
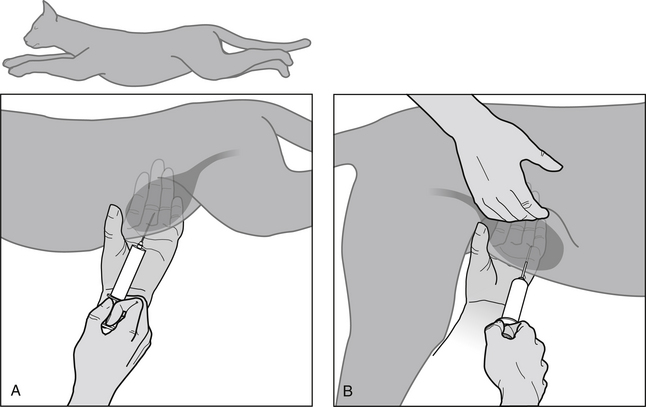
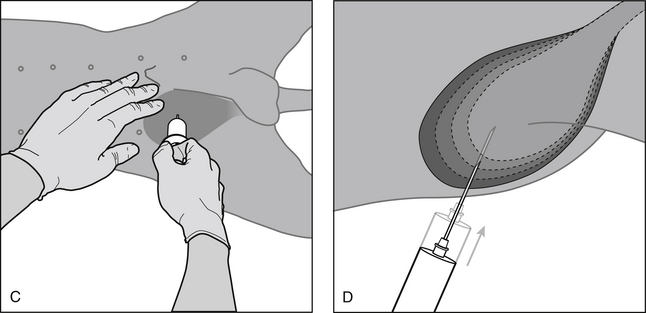
Technique (Figure 1-3)
Standard (Bladder Palpable and Can Be Stabilized)
(1) Restrain animal in lateral or dorsal recumbency (cat, small dog) or allow animal to remain standing (large dog).
(3) Clipping or wetting the hair with water to expose skin at puncture site is preferred by some operators but is not necessary. Do not disinfect the skin before cystocentesis because this carries a risk of contamination of urine by disinfectant and possible false negative culture results.
(4) Stabilize the bladder’s position by palpation. Compressing the bladder from the opposite side of the abdomen often facilitates its identification on palpation.
(5) Perform bladder puncture using a 22-gauge needle connected to a 6- or 12-mL syringe. Aim the needle toward the pelvic inlet to minimize bladder trauma because the bladder will decrease in size as urine is aspirated. Gentle negative pressure on the syringe during puncture allows urine to be aspirated immediately after penetration of the bladder lumen.
Blind (Bladder Not Palpable)
(2) Visualize a point on the ventral midline between the fourth and fifth teats and make needle puncture at this location.
(4) Choose a more cranial insertion site (i.e., closer to the fourth teat) if the bladder is very large.
(5) Push the viscera caudally with the fingers of one hand as necessary to allow a slight bulging of the bladder to be seen.
(7) Blind technique is successful in approximately 50% of attempts in dogs but is not recommended in cats because the position of the bladder in cats is considerably more variable than in dogs.
Disadvantages
a. Should not be attempted if cystotomy has been performed in the past week, if the animal has an atonic bladder, or if transitional cell carcinoma is likely present.
b. Potential risk of urine leakage if the bladder remains distended after the procedure.Leakage also may occur if the bladder wall is devitalized.
Complications
c. Seeding of tumor cells from transitional cell carcinoma potentially can occur, but the frequency and importance of this problem is not known.
e. Rarely a cat will collapse after cystocentesis, possibly due to catecholamine release, and this effect may be more severe in cats with underlying cardiovascular disease.
f. <25,000µ platelet count increases the risk for bleeding; <10,000µ platelet count is an absolute contraindication.
Performance of the Urinalysis
A. Examine fresh urine whenever possible to identify elements that are lost over time (e.g., cellular elements) and to avoid artifacts that develop in urine that is refrigerated or held at room temperature (e.g., crystal precipitation).
B. Collect urine specimens from dogs in the morning to obtain maximally concentrated urine. Timing of collection is not as important in cats.
C. Refrigerated urine should be warmed to room temperature before performing urinalysis because the color reactions of the chemical reagent pads on the dipstrips are temperature sensitive.
Interpretation of the Urinalysis
Physical Properties
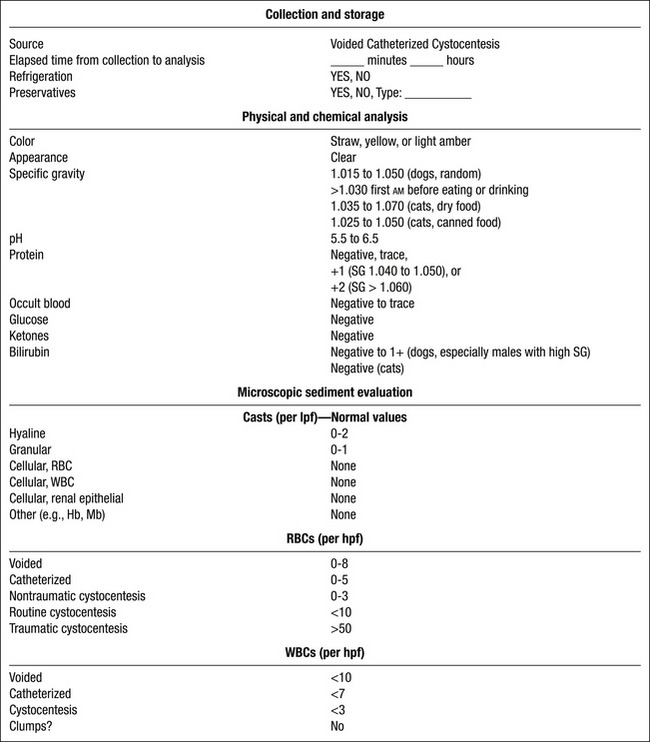
Color
Abnormal Urine Colors
Appearance
Specific Gravity
a. Urine specific gravity (USG) is the weight of the urine compared to that of an equal volume of water. It reflects both the total number of solutes and their weight (heavier molecules contribute more to USG than smaller ones).
c. USG is estimated by refractometry (refraction of light in solution is affected by the number and size of the particles in the solution).
(1) The refractometer should be temperature compensated for accurate and consistent estimates of USG.
(2) Dog and cat urine have different refractometric properties, and scales specifically developed for use in dogs or cats should be used for most accurate results.
(3) Optical and digital refractometers designed for veterinary use have scales that allow determination of USG values up to 1.060.
(5) USG should not simply be reported as >1.035 in dogs and cats. When the initial USG reading exceeds the refractometer’s scale, mix equal volumes of urine and distilled water and determine the USG. Multiply the numbers to the right of the decimal point by a factor of 2 to determine the actual USG.
d. Use the USG as a guide to interpret the relative concentration of abnormal elements or chemical constituents in the sample.
(1) 4+ (1000 mg/dL) proteinuria in a urine sample of 1.010 USG represents more severe proteinuria than 4+ (1000 mg/dL) proteinuria in urine of 1.045 USG.
e. USG should be determined before treatment with fluids, diuretics, corticosteroids, or other medications.
f. Repeated production of submaximally concentrated urine in dogs and cats usually indicates abnormal renal function (see Chapter 2).
g. The first urine of the morning is most likely to have the highest solute concentration. USG will vary based on diet moisture content, amount of water consumed, excess dietary solutes requiring renal excretion, renal function, and hydration status (see Chapter 15, Approach to Polyuria and Polydipsia).
(1) First urine of the morning should have USG >1.035 in cats consuming dry foods and >1.025 in cats consuming canned foods.
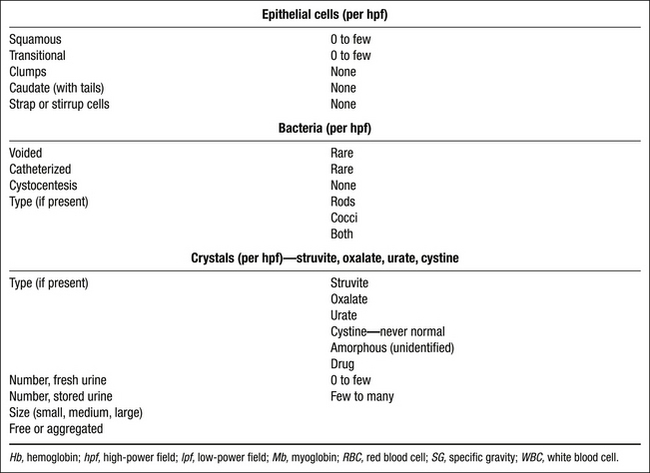
Chemical Properties
Dipstrip Evaluation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree