CHAPTER 5 Upper Respiratory Tract Aspergillosis
ETIOLOGY
Until recently, reports of feline URT aspergillosis have been limited, describing infections in a total of 13 cats.1–9 The affected cats presented with signs referable to sinonasal cavity infection (eight cases) or sino-orbital infection (five cases). The fungal pathogen often was not identified in these reports, because either fungal culture was negative or was not performed. In five cases in which fungal culture was positive, isolates were proposed to be Aspergillus spp. in four cases and Penicillium spp. in another.1,2,6,8,9 Identification to species level based on morphological and cultural characteristics was performed in only two cases, in which A. fumigatus and A. niger were documented.8,9
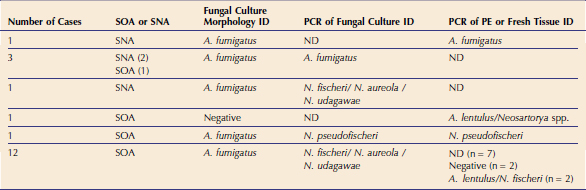
In 2006 three cases of feline URT aspergillosis were diagnosed at the authors’ institution. This prompted a search of our medical records, which identified only three cases in the preceding 19-year period (January 1986 to December 2005). Following publication of a description of the syndrome of SOA and a call for cases, we actively recruited 15 additional cases for prospective study between 2007 and 2008.10 Clinicopathological data from the resulting cohort of 21 cats from Eastern Australia provide exciting new information on this disease in cats.11 Fungal pathogens were cultured readily. Culture was attempted in all 21 cases and was positive in 20. Sabouraud’s dextrose agar was used with addition of gentamicin and chloramphenicol when bacterial contamination of diagnostic samples was suspected. Cultures were incubated at 37° C and 28° C. On the basis of colony morphology, A. fumigatus was the most common isolate identified in this cohort. However, it is important to understand that the correct identification of some species of fungi belonging to Aspergillus section Fumigati can not be achieved using morphological and cultural characteristics alone.12,13 Therefore molecular diagnostics were performed on the fungal cultures and/or on formalin-fixed paraffin embedded (PE) or fresh tissues from affected cats to confirm the identity of the isolates. A panfungal polymerase chain reaction (PCR) that amplifies the internal transcribed spacer 1 (ITS1) region of the ribosomal DNA gene cluster between the 18S and 5.8S rRNA genes was used for PE or fresh tissues. A second panfungal PCR that targets a larger region of the rDNA gene cluster including the ITS1, 5.8S gene, and ITS2 regions was carried out on material obtained from fungal culture. These regions of the fungal genome were chosen because they are multicopy (⩾ 100 copies in the fungal genome) and because they contain highly variable regions facilitating species identification in some cases. PCR products were sequenced and compared with those in the GenBank database.14,15
Significantly, results of these molecular analyses indicated that the majority of feline isolates that had been identified as A. fumigatus based on culture morphology were actually Neosartorya spp. (Table 5-1). In most cases, it was not possible to classify the fungal pathogen further using the panfungal PCR because of limited sequence variation in the ITS1 region; for example, it was not possible to distinguish between N. udagawae, N. fischeri, and N. aureola. In one case, N. pseudofischeri was identified from PCR of both culture material and fresh tissue with 100 per cent identity to sequences in the GenBank database. In four cats, A. fumigatus was identified using both fungal colony morphology and PCR of culture material or tissue. In three of these cats, disease was confined to the sinonasal cavity. PCR of fungal culture material and/or tissue obtained from seven canine SNA cases also was carried out and identified A. fumigatus in all cases.14 These findings suggest that A. fumigatus possibly may be associated with less invasive disease. Our experience has demonstrated that, unlike SNA in dogs, the most common isolates in URT aspergillosis in cats are Neosartorya spp. and that molecular diagnostic tests are required for the correct identification of these fungal pathogens.
Table 5-1 Comparison of Fungal Culture Morphology versus PCR of Fungal Culture and/or Formalin-Fixed Paraffin-Embedded Tissues (PE) or Fresh Tissue for Fungus Identification (ID) in 18 Cases
CLASSIFICATION OF FUNGAL PATHOGENS
Both Aspergillus spp. and Neosartorya spp. are implicated as fungal pathogens in feline URT aspergillosis. These two genera are very closely related, and a brief review of their taxonomy is pertinent. Both are classified within Aspergillus section Fumigati. These ubiquitous, filamentous, saprophytic ascomycetes are distributed primarily in soil and decaying vegetation.16 Confusion may arise over nomenclature because fungi can exist in different physical states corresponding to different stages of the life cycle; the anamorphic, asexual stage typically is moldlike and bears conidia (spores), whereas the teleomorphic, sexual stage is characterized by the production of fruiting bodies (cleistothecia) containing ascospores. The total organism is referred to as the holomorph. Some Aspergillus species are strictly anamorphic and therefore reproduce by asexual means only. Teleomorphic species within Aspergillus section Fumigati have been assigned to the genus Neosartorya. Aspergillus section Fumigati currently includes 9 strictly anamorphic Aspergillus spp. and 24 Neosartorya spp.16,17 Aspergillus fumigatus, previously thought to be strictly anamorphic, was shown recently to have a fully functional sexual reproductive cycle that leads to the production of cleistothecia and ascospores. Its teleomorph is Neosartorya fumigata.17a
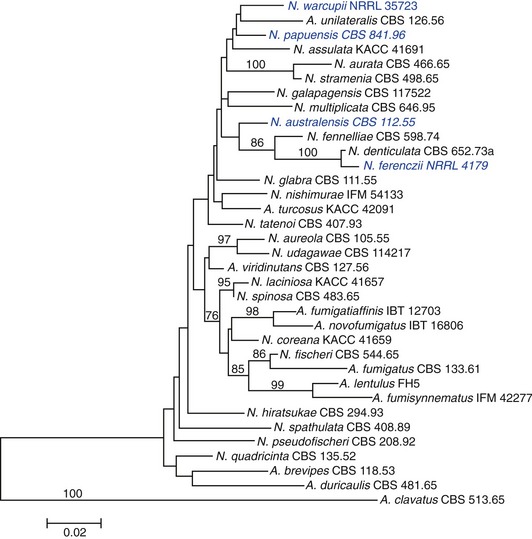
Species implicated in feline URT aspergillosis are listed in Table 5-2. Neosartorya spp. can exist in both anamorphic and teleomorphic states. The anamorphic states of some Neosartorya spp. can be mistaken for A. fumigatus, if identification is based on phenotypic characteristics such as thermotolerance and conidiophore morphology.13,14 Phenotypic identification of Neosartorya spp. can be achieved partially from its teleomorph, including morphology of ascospores. However, induction of the teleomorphic state of Neosartorya spp. to produce these fruiting bodies can be achieved only under specific growth conditions with specialized media. Even under optimal conditions, some species may not produce fruiting bodies in the laboratory.14 Because of these limitations for species identification using morphological criteria alone, a polyphasic taxonomic approach is recommended for identification of species within Aspergillus section Fumigati. This involves a combination of macromorphology and micromorphology, extrolite (fungal metabolite) profiles, and PCR determination of gene sequences including β-tubulin, calmodulin, ITS, and actin.16,18 The phylogenetic relatedness of species belonging to Aspergillus section Fumigati, including pathogens implicated in feline URT aspergillosis based on β-tubulin sequence data, is depicted in Figure 5-1.
Table 5-2 Species Belonging to Aspergillus Section Fumigati that Have Been Implicated in Feline Sinonasal and Sino-orbital Aspergillosis16
| Strict Anamorphic Species | Teleomorph |
|---|---|
| None |
| Anamorph | Teleomorphic Species |
|---|---|
EPIDEMIOLOGY AND RISK FACTORS
No age or sex predilection for feline URT aspergillosis is apparent. Disease has been reported in 15 females and 18 males; most were neutered. The median and mean age at diagnosis was 6 years, with a range from 18 months to 13 years.1–911 An intriguing finding is the preponderance of brachycephalic cats affected; sixteen of 34 cases were brachycephalic, including eight Persians and six Himalayans. This contrasts starkly with canine SNA, in which dolichocephalic or mesocephalic breeds are typically affected.19 The basis for this potential brachycephalic breed association in cats is not clear. Impaired drainage of URT secretions due to brachycephalic conformation may be important. Certainly, decreased sinus aeration and drainage of respiratory secretions secondary to infection, polyps, and allergic rhinosinusitis have been identified as risk factors for invasive SNA aspergillosis in human beings.20 It is likely that additional risk factors are present in brachycephalic cats because brachycephalic dogs are underrepresented for SNA. These factors may include heritable defects in mucosal immunity or common environmental factors such as antecedent URT infection. Previous viral URT infection by feline calicivirus (FCV) or feline herpesvirus-1 (FHV) has been suggested as a possible risk factor for aspergillosis in cats.7 In particular, chronic FHV infection can alter sinonasal cavity architecture severely because of turbinate lysis secondary to intense inflammation, resulting in altered local mucosal defense mechanisms. Six of 20 cats in one study had a history of chronic recurrent rhinosinusitis before aspergillosis was diagnosed, and FHV infection was confirmed by PCR in one cat.11 Other possible risk factors identified were craniofacial trauma, diabetes mellitus, and a grass seed foreign body. There is no evidence currently of a retroviral association with feline URT aspergillosis; of 19 cats with aspergillosis tested for feline leukemia virus (FeLV) antigen, only one was positive, and all 19 cats tested for feline immunodeficiency virus (FIV) were seronegative.3,5–9,11 Therefore it appears that URT aspergillosis in cats occurs in apparently systemically immunocompetent individuals, some of whom have identifiable breaches in local defense mechanisms.
Information on the prevalence and geographic distribution of feline URT aspergillosis is incomplete but is likely to be forthcoming now that the syndrome of SOA has been described.10 The apparent increased prevalence at our institution is likely a consequence of increased recognition of cases. Cases have been reported most commonly in Eastern Australia (including 12 cats domiciled in New South Wales, two in Victoria, and seven in Queensland) and the United States, with individual cases from Switzerland and the United Kingdom.1–3,5–9,11
PATHOGENESIS
Aspergillus spp. and Neosartorya spp. are ubiquitous and their spores are inhaled readily. Aspergillus spp. can cause localized sinusitis in immunocompetent human beings or severe systemic disease as an opportunistic pathogen in immunocompromised human patients. Neosartorya spp. have been associated only rarely with disease; isolated cases of invasive pulmonary infection, osteomyelitis, endocarditis, peritonitis, and mycotic keratitis have been described in immunocompromised human beings.13 The difficulty in distinguishing A. fumigatus from Neosartorya spp. using morphological criteria is likely to have resulted in underestimation of the frequency of disease caused by Neosartorya spp.13
Compared to SNA in dogs in whom fungal infection generally is confined to the sinonasal cavity, infection in cats appears to have a greater tendency to local invasion, with extension to involve the orbit. Of the 34 reported cases, 14 cats had SNA and 20 had SOA.* A brief consideration of mycotic rhinosinusitis syndromes in human beings has comparative significance because these syndromes can be either invasive or noninvasive. Invasive mycoses include acute (necrotizing) invasive fungal sinusitis, chronic invasive fungal sinusitis, and chronic granulomatous fungal rhinosinusitis. Noninvasive infections include fungal ball or sinus aspergilloma, allergic fungal sinusitis, and chronic erosive noninvasive fungal sinusitis.21–24 Canine SNA bears many similarities to chronic erosive noninvasive fungal sinusitis in human patients and recently has been proposed as a model for studying the immunopathogenesis of disease in people.24 A. fumigatus is the most common pathogen in dogs, although A. niger, A. nidulans, and A. flavus are implicated occasionally. A. fumigatus or A. flavus are isolated most commonly from human patients.19,22,24 Neither dogs nor human beings with this type of SNA are immunocompromised systemically. Fungal hyphae do not invade the nasal mucosa and typically are present within overlying superficial necrotic plaques. There is a mixed mononuclear inflammatory response within the often ulcerated nasal mucosa that contains lymphocytes and plasma cells predominantly, with some macrophages.19,21,22
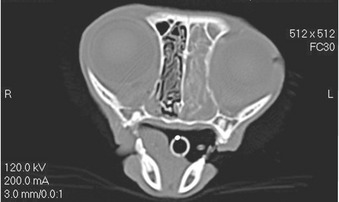
Like SNA in dogs, infection in cats starts almost certainly in the sinonasal cavity. It is likely that SNA and SOA in cats represent a spectrum of disease, with SOA being a manifestation of more chronic, invasive infection and progression from SNA. Progression from SNA to SOA has been documented in individual cases.6 However, not all cases of SNA progress to SOA. Virulence factors of the fungal pathogen, including toxic secondary metabolites, are likely to be involved. Gliotoxin is considered an important virulence factor in invasive aspergillosis in human patients. All 20 cases of SOA in cats showed evidence of sinonasal cavity involvement on diagnostic imaging or at necropsy.* The orbital lamina, situated between the orbit and frontal sinus, is the most likely region where extension of infection from the sinonasal cavity to the orbit occurs. Computed tomographic (CT) examination of the skull in cases of SOA frequently identifies punctate areas of lysis in the orbital lamina (Figure 5-2). In one case of SOA, a fistula was identified at surgery in the dorsomedial aspect of the orbit, communicating with the ipsilateral frontal sinus.11 Systemic dissemination of disease is rare. A single case of feline SOA with concurrent pulmonary involvement has been reported. The retroviral status of that cat was unknown.1
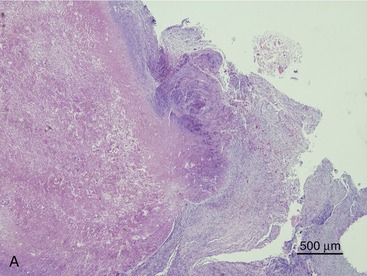
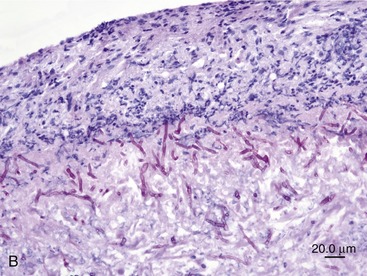
SOA in cats bears a striking clinical and histological resemblance to chronic granulomatous sinusitis in people. The latter is an invasive mycosis caused by A. flavus or A. fumigatus that occurs in immunocompetent human beings subjected to hot, dry environmental conditions and poor hygiene. Most cases have been reported in Asia and Africa, predominantly in agricultural workers. The nasal cavity or sinuses invariably have been implicated as the primary site of infection following inhalation of fungal spores. A fungal granuloma forms within the sinuses. These granulomas are relatively avascular, consisting of a highly cellular inflammatory infiltrate of epithelioid macrophages (“giant cells”), histiocytes, plasma cells, and fungal hyphae. They may be necrotic or fibrotic and tend to invade contiguous structures such as the orbit or brain. Unilateral exophthalmos is common at presentation.20,23 The histological features of URT aspergillosis in cats are not fully characterized. Both lymphoplasmacytic and suppurative noninvasive rhinitis have been described for infections confined to the sinonasal cavity.7,8 SOA is clearly invasive, and we have found evidence of submucosal infection in the sinonasal cavity of affected cats.11 In feline SOA, the granulomas are characterized by a necrotic center containing fungal hyphae. This is surrounded by granulation tissue that may be fibrous, infiltrated with neutrophils, lymphocytes, plasma cells, and eosinophils.1,2,9 Commonly, we have seen retrobulbar granulomas surrounding the optic nerve. They typically feature a necrotic center containing branching septate PAS-positive fungal filaments and an inflammatory infiltrate of sheeting epithelioid macrophages with variable numbers of lymphocytes, plasma cells, and neutrophils extending into a zone of peripheral fibrosis (Figure 5-3). These fungal granulomas are well vascularized and in some cases are accompanied by heavy infiltrates of eosinophils.
HISTORY AND CLINICAL SIGNS
SINONASAL ASPERGILLOSIS
In dogs, the triad of muzzle pain, profuse mucoid to hemorrhagic chronic nasal discharge and depigmentation, and crusting or ulceration of one or both nares is highly suggestive of SNA. In cats, presenting signs of SNA are less specific. Most affected cats will have a history of sneezing and a unilateral or bilateral serous to mucopurulent nasal discharge that usually can be detected on physical examination (Figure 5-4). Intermittent epistaxis occurs in 40 per cent of cats with SNA. Interestingly, and in contrast to dogs, neither nasal depigmentation nor ulceration has been documented to date in cats with SNA. A discharging sinus or soft tissue mass may be identified overlying the frontal sinus or the nasal bone as a result of bony lysis and fungal proliferation (Figure 5-5). Facial distortion may be a feature.
Owners should be questioned specifically regarding any URT noise because this may be subtle, and the pattern of respiration should be evaluated carefully on physical examination. If it can be established that the cat has a stertor, at least part of the disease process can be localized to the caudal nasal cavity and/or nasopharynx. The stertor in SNA may result from excessive nasal secretions and/or a mass lesion in the caudal nasal cavity and/or nasopharynx (Table 5-3).
Table 5-3 Spectrum of Clinical Signs in Cats with SNA and SOA
| Clinical Presentation | Clinical Signs |
|---|---|
| Sinonasal aspergillosis | |
| Sino-orbital aspergillosis |
SINO-ORBITAL ASPERGILLOSIS
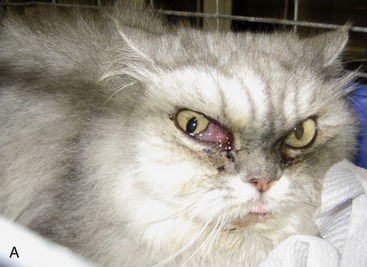
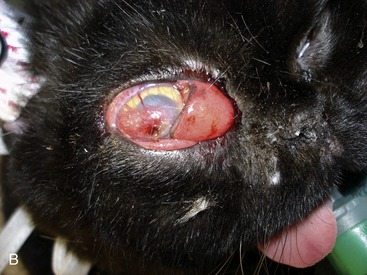
Although most cats with SOA have a history of sneezing or nasal discharge in the 6 months prior to presentation, it is important to note that, at the time of presentation, nasal signs may be subtle or absent. Rather, cats with SOA present typically with a constellation of clinical signs referable to invasive expansion of a fungal granuloma in the ventromedial orbit. These include unilateral exophthalmos with third eyelid prolapse, exposure keratitis and conjunctival hyperemia (Figure 5-6), and a mass or ulcer in the ipsilateral pterygopalatine fossa behind the last molar tooth (Figure 5-7). Stertor also is common. In the majority of affected cats, exophthalmos is unilateral, but in severe, chronic infections, bilateral exophthalmos can occur. Pain on opening the mouth typically is not present. In one cat with confirmed fungal sinusitis, marked unilateral exophthalmos resulted from retrobulbar myofascitis rather than from a fungal granuloma.5 Invasion through the palatine bone can cause ulceration of the hard palate (Figure 5-8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree