Chapter 57 Updated Vaccination Recommendations for Carnivores
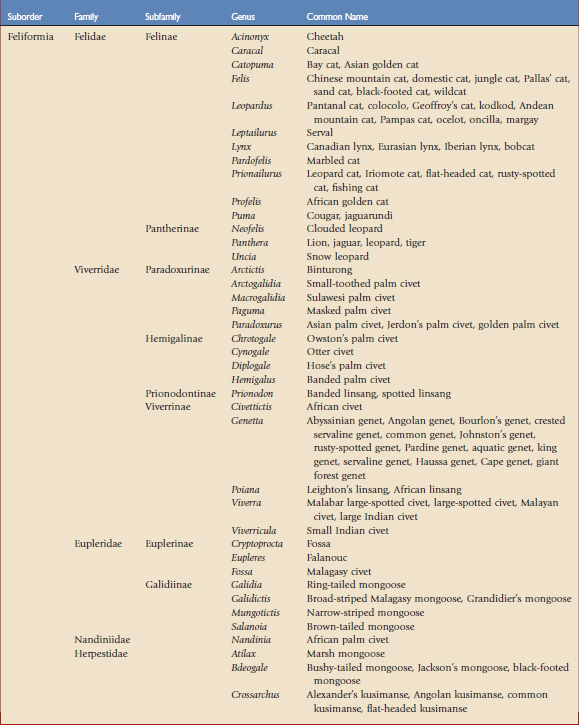
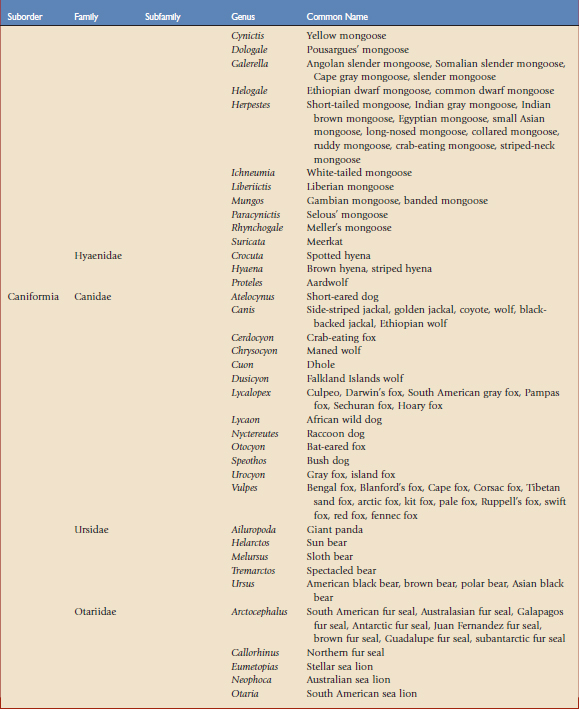
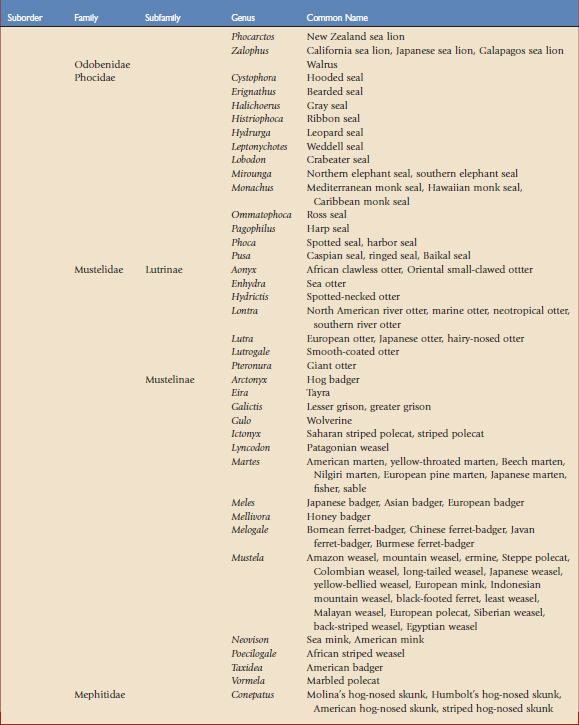
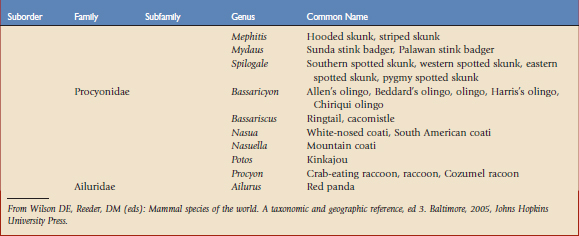
Using the taxonomic classifications presented by Wilson and Reeder,17 the order Carnivora is divided into two suborders, Feliformia and Caniformia (Table 57-1). Knowing which species are more catlike or more doglike may help predict disease susceptibility when published data are lacking. Commercial vaccines have been developed for use in domestic species, and using them in other carnivores constitutes extralabel use. Although modified live and killed virus vaccines dominate the market, third-generation products are now complementing and in some cases replacing them.4,8,11 This new technology should have a positive impact on current practices by improving the safety and efficacy of vaccines used in nondomestic carnivores.
Guidelines that have global application for the vaccination of cats and dogs have been compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association.18 These guidelines were based on those provided by the American Animal Hospital Association (AAHA) Canine Vaccine Task Force and the Feline Vaccine Advisory Panel of the American Association of Feline Practitioners.8,9 The guidelines are also consistent with those of the European Advisory Board on Cat Diseases and the South African Veterinary Council.4,14 The information in this chapter is a condensed version taken directly from these guidelines, with special attention as to how they might be applied to nondomestic carnivores. This information is subject to change in light of developments in research, technology, and experience.
Core Vaccines: Universally Recommended
The VGG recommends that all cats and dogs benefit from vaccination. Vaccination protects the individual and provides optimum herd immunity by reducing the number of susceptible animals in the regional population and decreasing disease prevalence. Specific recommendations are based on the concept of core vaccines. Core vaccines are those that every cat or dog, regardless of circumstances, should receive. Core vaccines protect animals from severe life-threatening diseases that have global distribution. Vaccination programs should include only those vaccines that the animal truly needs because all vaccines have the potential to cause adverse reactions.18
The core vaccines for felids are those that protect from feline parvovirus (panleukopenia) (FPV), feline calicivirus (FCV), and feline herpesvirus (FHV). Rabies virus is included in this core group in areas of the world in which rabies is endemic. With the exception of Australia, Great Britain, Japan, and some islands, rabies is present worldwide.9 Parenteral killed FPV vaccine is usually preferred because vaccine-associated disease can occur if using a modified live (ML) product, although this has not been documented for ML FPV vaccine. Immunity takes 3 or more weeks to develop after a first dose of killed vaccine and at least 1 week after the second dose. This is in contrast to the ML FPV, which provides long-lasting immunity in approximately 3 days in cats older than 16 weeks. Products containing ML FPV also contain ML FCV and ML FHV. Similarly, products containing killed FPV contain killed FCV and FHV.13
Killed vaccines can be used in pregnant animals and should be used in disease-free collections. Using ML products (including the intranasal FPV-FCV-FHV vaccine) can introduce calicivirus and herpesvirus into collections that were previously free of these conditions and is not recommended for nondomestic carnivores. The protection afforded by the FCV and FHV vaccines (either killed or ML) will not provide the same efficacy of immunity as seen with the FPV vaccines. When vaccination does not prevent infection with FCV or FHV, systemic and local cell-mediated and humoral immunity play important roles in preventing or reducing the severity of disease.9 Although the FCV vaccines have been designed to produce cross-protective immunity against severe clinical disease, there are multiple strains of FCV. It is therefore possible for infection and mild disease to occur in the vaccinated animal.
Virulent systemic calicivirus (VSCV) has recently been described. Vaccination with current vaccines does not protect felids against field infections, but some protection has been shown experimentally.13,18 There is no FHV vaccine (ML or killed) that can protect against infection with virulent strains of herpesvirus. Virulent strains of herpesvirus will become latent and may be reactivated during periods of severe stress for the life of the felid. The reactivated virus may cause clinical signs in the vaccinated animal or the virus can be shed to susceptible animals and cause disease. This is seen most frequently in captive cheetahs. Vaccination during pregnancy can help protect kittens by prolonging maternally derived antibody (MDA).9 Cats may become infected with canine parvovirus and certain strains may cause signs of panleukopenia in cats.7,15 Conventional FPV vaccines have been shown to protect against these canine parvoviruses, but there is a report of a cheetah vaccinated with a killed FPV vaccine that developed canine parvovirus infection (CPV-2b) and gastrointestinal disease.3,6,16
The core vaccines for canids are those that protect from canine distemper virus (CDV), canine adenovirus (CAV), and canine parvovirus (CPV). Rabies virus is included in this core group in areas of the world in which rabies is endemic. In areas or facilities in which CDV is not endemic in domestic or wild susceptible species, ML vaccines should not be used. The risk of introducing a virus into a host population is unacceptable.11,18 Susceptible wild carnivore species do shed virus following ML CDV vaccine administration and may develop disease. This has been reported in the red panda, black-footed ferret, European mink, gray fox, kinkajou, South American bush dog, and maned wolf.2 The ML CDV products available contain virulent strains and are for use in domestic dogs only. A poxvirus recombinant canine distemper vaccine (rCDV) is available in many countries and is considered the vaccine of choice for nondomestic carnivores to protect against vaccine-induced disease and natural infection. This recombinant vaccine has the added advantages of being safe in younger animals and more effective for immunizing carnivores with MDA. In the absence of MDA, rCDV vaccine provides immunity immediately after vaccination.8,12 The rCDV vaccine is currently available as a monovalent product or combined with ML CPV and ML CAV, or with ML CPV, ML CAV, and canine parainfluenza virus (CPI). CPI is considered an optional vaccine (see later).
There are four genotypes of canine parvovirus that are recognized worldwide—CPV-2, CPV2a, CPV-2b, and CPV-c—and all genotypes are antigenically comparable. This means that vaccination with any one genotype provides protective immunity against all other genotypes.9,18 CPV is hypothesized to be a natural genetic mutation of FPV that first emerged in dogs in 1978.6,7 CPV strains can replicate in both canine and feline cells, but FPV has been shown to only replicate efficiently in feline cells. There are only a few killed CPV vaccines available and are less effective than the ML vaccines. In addition, they are often combined with a ML CDV vaccine. Although killed vaccines are preferable for use in wild or nondomestic species, this killed product may not be the best choice if it is part of a polyvalent vaccine that contains ML CDV for the reasons noted earlier. Canids are most susceptible to severe disease caused by CPV infection during the first year of life. Each institution should decide whether they could effectively isolate pups to prevent introduction of disease or whether the benefits of using a ML CPV product outweigh the risks. ML CPV vaccination will result in shedding of virus, and this virus could potentially revert to virulence, as well as infect other individuals or other species. Once in the environment, the virus can remain infectious for 1 year or longer.8 Similar to ML FPV vaccines, ML CPV vaccines provide immunity 3 days after vaccination. When CPV first appeared, FPV vaccines were used to provide some protection until a more specific vaccine was manufactured.15 It is not known whether a killed FPV monovalent vaccine will protect against CPV in nondomestic carnivores but recent studies have suggested that it may offer some protection.3 Alternatively, one could consider not revaccinating canids with a positive antibody titer to CPV postvaccination using a ML product, because life-long immunity should result.12 Experimental canine DNA vaccines have been developed for CPV-2 and hold promise for the future. In contrast to ML CPV vaccines, ML CAV vaccine virus has not been shown to revert to virulence in back passage studies. ML CAV-2 containing vaccines are the most commonly available products. They will prevent infectious canine hepatitis (ICH) caused by CAV-1 and reduce the signs of respiratory disease caused by CAV-2. They also do not cause the adverse reaction commonly seen with CAV-1 vaccines, known as allergic uveitis, or blue eye.
MDA significantly interferes with the efficacy of most core vaccines currently available, with the exception of rCDV vaccine. Because the level of MDA varies among litters, young carnivores should receive three doses of vaccine. These repeated injections in the first year of life do not constitute boosters but rather are an attempt to induce a primary immune response. In general, passive immunity will have waned by 8 to 12 weeks to a level that allows for active immunization. The age at which to begin the vaccination series can vary, but vaccinations are typically begun between 6 and 9 weeks of age and then are readministered at 2- to 4-week intervals until the animal is 14 to 16 weeks of age; 16 weeks is usually preferable to ensure that the waning of maternal antibody is complete.8,9,12,13,18 Starting the immunizations as early as 6 weeks may be appropriate in situations of high risk (e.g., FHV in cheetahs) and questionable MDA status. In situations in which only one vaccine can be administered, this should be done when the animal is immunologically capable of responding—that is, at the age of 16 weeks or older. After an initial series of three vaccines, the animal should receive a booster in 12 months. The initial series and this booster are referred to as the primary vaccination course. The booster at 12 months also ensures immunity for carnivores that did not adequately respond to the first series of vaccines. Core vaccines are given no more frequently than every 3 years thereafter. There are reports in the literature that suggest that the duration of immunity is at least 3 years when killed products are used.4,8,10 A notable exception is that an annual booster is required for the recombinant virus, nonadjuvanted, canarypox-vectored rabies vaccine labeled for use in domestic cats. For this reason, the killed rabies vaccine is often chosen, despite concerns with the use of adjuvants. Primary rabies vaccination should occur at 12 to 16 weeks of age, with revaccination 1 year later. Annual or triennial vaccination should follow, depending on the type of vaccine used and applicable legal requirements. There is a push towards marketing vaccines with an extended duration of immunity (DOI). This will reduce the unnecessary administration of vaccines, thereby further improving vaccine safety.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree