K. Gary Magdesian
Update on Antimicrobial Selection and Use
Antimicrobial use in equine practice is constantly evolving. Judicious antimicrobial use is critical to minimizing selection pressure for antimicrobial resistance among bacteria. Thoughtful, indication-based prescription and selection should be at the forefront of antimicrobial use in equine medicine. The goal of this chapter is to provide a review of new findings in antimicrobials available for use in horses, and is meant to update the chapter from the previous edition. A comprehensive review of all antimicrobials available for use in equine practice is beyond the scope of this chapter.
Cephalosporins: New Drugs and Novel Administration
Ceftiofur Sodium
Ceftiofur is a third-generation cephalosporin approved for use in horses by the intramuscular (IM) route only (the label dosage for streptococcal infections is 2.2 to 4.4 mg/kg intramuscularly every 24 hours). Recently, the pharmacokinetics of ceftiofur was studied after intravenous (IV) and subcutaneous (SC) administration. The IV and SC routes yielded concentrations of ceftiofur and metabolites similar to those achieved after IM administration. The SC route is convenient for use in neonatal foals that do not have IV catheters in place and is often better tolerated than the IM route. The author administers ceftiofur at dosages up to 5 to 10 mg/kg intramuscularly every 12 hours in neonatal foals, with the goal of achieving concentrations effective against bacteria that typically have higher minimum inhibitory concentration (MIC) values than Streptococcus spp and are commonly associated with neonatal sepsis, especially gram-negative enteric microbes. These dosages should not be used in adult horses because of the risk for inducing antimicrobial-associated colitis.
Ceftiofur Crystalline Free Acid Suspension
A new formulation of ceftiofur, ceftiofur crystalline free acid,1 was recently approved for use in horses. This is a suspension of ceftiofur labeled for the treatment of lower respiratory infections caused by Streptococcus equi subsp zooepidemicus. Ceftiofur sodium2 has a label dosage of 2.2 to 4.4 mg/kg intramuscularly once a day for up to 10 days, and a maximum of 10 mL should be administered per injection site. In contrast, the crystalline free acid form is dosed at 6.6 mg/kg intramuscularly, with two doses administered 4 days apart to provide for a 10-day duration of antimicrobial coverage.
The crystalline free acid form is continuously released after IM injection. The first dose provides plasma concentrations above the MIC of S equi zooepidemicus for at least 4 days, whereas the second dose does so for 6 days. The label calls for injection of a maximum of 20 mL per site, but the incidence of injection site swelling and soreness decreases when no more than 10 mL is injected per site. The average plasma concentrations of the crystalline free acid formulation at this dosage are similar to those achieved by a dose of 2.2 mg/kg of ceftiofur administered IM once daily.
Contrary to a somewhat common misconception, ceftiofur crystalline free acid is not meant to be used as a broad-spectrum antimicrobial. Rather, it is specifically indicated for bacteria with an MIC of 0.25 µg/mL or less for ceftiofur, such as Actinobacillus spp, Pasteurella spp, and Streptococcus spp. Bacteria that would not be considered susceptible at this concentration include Staphylococcus and many gram-negative enteric bacteria. Corynebacterium pseudotuberculosis has an MIC(90) of 1 µg/mL, and infections by this pathogen warrant the higher concentrations associated with higher doses of ceftiofur sodium, rather than those provided by ceftiofur crystalline free acid.
A recent study revealed that three additional weekly doses of ceftiofur crystalline free acid beyond the day 0 and day 4 doses were well tolerated and resulted in plasma and pulmonary concentrations that were above the MIC (<0.12 µg/mL) of respiratory pathogens S equi zooepidemicus, Pasteurella spp, and Actinobacillus spp.
Potential adverse effects of ceftiofur crystalline free acid suspension include swelling and sensitivity at the site of injection. This can be reduced by administering no more than 10 mL per site. Diarrhea is also a potential adverse effect, but this risk appears to be low. In the U.S. Food and Drug Administration pharmacokinetic study, some horses in both the Excede and Naxcel groups developed foot pain, heat, and pulses. However, researchers believed that concrete slabs in pens and dietary changes associated with the study may have contributed to this. To the author’s knowledge, this has not been observed in clinical practice.
Fourth-Generation Cephalosporins
Fourth-generation cephalosporins have been studied for use in foals and are widely used in the United Kingdom (cefquinome). Cefepime is an example of a drug with expanded gram-positive and gram-negative coverage, and it is resistant to β-lactamase enzymes. It is similar to ceftazidime, a third-generation cephalosporin, in its activity against Pseudomonas spp. Cefepime has a wide volume of distribution and is effective in treating meningitis because of its ability to penetrate the blood-brain barrier well. Shortcomings of cefepime include lack of activity against methicillin-resistant Staphylococcus aureus and the enterococci. It is only variably effective against anaerobic bacteria. The recommended dosage of cefepime in foals is 11 mg/kg intravenously every 8 hours. Cefepime should not be administered to adult horses because of the high risk for gastrointestinal disturbances observed under experimental conditions.
Cefquinome is another fourth-generation cephalosporin that is licensed in the United Kingdom for use in foals with septicemia and horses with respiratory tract disease. The recommended dosage is 1 mg/kg intravenously or intramuscularly every 12 hours (for foals with septicemia) or every 24 hours (for adults with respiratory tract disease).
Another relatively new finding in cephalosporin pharmacology in horses is the use of cefpodoxime, an oral third-generation cephalosporin, in neonatal foals. Like cefotaxime and ceftiofur, cefpodoxime has potent antibacterial activity against numerous gram-positive and gram-negative bacteria. On the basis of the pharmacokinetics of cefpodoxime in foals, an oral dosage of 10 mg/kg every 6 to 12 hours is recommended. Dosing every 6 to 8 hours is suggested for microbes with higher MIC values, including Escherichia coli (75% of equine E coli isolates) and Salmonella spp. Agents with lower MIC values, such as Streptococcus spp, Klebsiella spp, and Pasteurella spp, could be treated every 12 hours. Cefpodoxime is ineffective against Pseudomonas spp, Enterococcus spp, and Rhodococcus equi isolates in horses. The drug distributes well to synovial and peritoneal fluids and concentrates in urine, but it does not enter cerebrospinal fluid (CSF). Although cefpodoxime is absorbed in adult horses, two of six animals developed signs of colic in one experimental study.
Continuous-Rate Infusion of β-Lactam Drugs
Continuous-rate infusion (CRI) administration of antimicrobials is being used with increasing frequency in both human and veterinary medicine. This dosing regimen is intended to optimize the pharmacokinetics of antimicrobials on the basis of their specific pharmacodynamic properties. It is particularly suited for antimicrobials with time-dependent activity, such as β-lactam antimicrobials. With β-lactam drugs such as the penicillins and cephalosporins, efficacy is dependent on the duration of time for which plasma concentrations remain above the organism’s MIC. Continuous maintenance of plasma concentrations above the MIC therefore offers an advantage over intermittent administration for time-dependent antimicrobials. Continuous administration of an antimicrobial is particularly advantageous in immunocompromised animals, such as neutropenic and hypoglobulinemic neonatal foals with sepsis. The author uses CRI administration of β-lactam antimicrobials in horses with severe sepsis or septic shock and whenever fluid lines and automated pumps can be maintained, such as in foals and horses that are recumbent or confined to their stall.
Two methods of dose calculation are possible for determining CRI rates in clinical practice. The first is to administer the same total daily dose during continuous infusion as would be administered during intermittent infusion. For example, if a drug is conventionally administered at a dosage of 20 mg/kg every 8 hours, the CRI dosing rate would be 2.5 mg/kg per hour to maintain the same total daily dose.
Alternatively, doses can be determined on the basis of pharmacodynamic considerations of the drug. With β-lactam antimicrobials, the goal is to maintain plasma free-drug concentrations at four times the MIC of the offending microorganism for as long as possible in between doses. Because they are time dependent rather than concentration dependent, β-lactam drugs reach their maximum killing rate at concentrations no higher than four times the MIC. The desired concentration for β-lactam drugs is therefore four times the MIC of the bacterial agent, optimally maintained continuously. The dosing rate is calculated as the product of drug clearance and the desired average (steady state) plasma concentration of drug. For example, if the clearance of a drug is known to be 0.2 L/kg per hour and the desired steady-state concentration is 1 µg/mL (4 × MIC of the offending agent: in this example, MIC is 0.25 µg/mL), the CRI dosing rate is as follows:
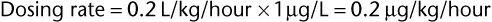
The clearance of many drugs is known from pharmacokinetics studies; for drugs with unknown clearance (Cl), it can be determined from published values of half-life (t1/2) and volume of distribution (Vd):
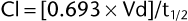
The stability of the administered antimicrobial in solution, both at room temperature and under refrigeration, is an important consideration when selecting β-lactam drugs for CRI administration because the syringe or bag containing the antimicrobial is usually maintained at room temperature. A programmable automated pump should be used to determine accurate infusion rates. If drugs are stable for less than 8 hours at room temperature, it is important that solutions be prepared and changed frequently; otherwise, they should be administered with conventional intermittent dosing. Ceftazidime, cefotaxime, cefepime, and ticarcillin disodium3 are stable for at least 24 hours at room temperature. Sodium and potassium penicillin and cefazolin are stable for 24 hours once reconstituted, whereas the stability of ampicillin varies with the concentration of the reconstituted solution and should be verified from the product label. After reconstitution, ceftiofur is stable for 12 hours at room temperature, but it should be protected from light.
A recent study evaluated the pharmacokinetics of ceftiofur administered as a CRI to foals. On the basis of study results, the authors predicted that a bolus loading dose of 1.26 mg/kg followed by a CRI of 2.86 µg/kg/minute would maintain plasma concentrations of desfuroylceftiofur (DCA) of at least 2 µg/mL. This is equal to a daily dose of 5.4 mg/kg/day. For bacteria with higher MIC values (up to 4 µg/mL for DCA), higher doses may be required. When switching from a CRI to intermittent bolus administration as the foal improves, the bolus dose should be started 12 hours after the end of the CRI.
Another study compared CRI and intermittent bolus administration of cefotaxime in 1-day-old pony foals. The dosage is 40 mg/kg intravenously every 6 hours at the standard intermittent dosing protocol, and for CRI administration it is 160 mg/kg/day (6.7 mg/kg/hour) after an initial loading bolus dose of 40 mg/kg. This maintains the same total daily dose of cefotaxime. With CRI dosing, synovial fluid concentrations are significantly higher than those achieved by intermittent dosing. In addition, mean plasma concentrations are constantly maintained above the MIC of most susceptible pathogens (at approximately 16 µg/mL), whereas intermittent dosing allows for concentrations to get as low as 0.78 µg/mL at 6 hours after dosing. Many Pseudomonas spp, methicillin-resistant Staphylococcus spp, and some E coli isolates are not susceptible to cefotaxime, even with CRI dosing. For time-dependent antimicrobials such as cephalosporins, CRI dosing is therefore advantageous in optimizing the duration by which drug concentrations exceed the MIC of common equine pathogens. Cefotaxime is compatible with 0.9% saline and is stable for at least 24 hours at room temperature after mixing as long as it is protected from light.
Macrolide Antimicrobials
Erythromycin has been used for treatment of R equi infection in foals for many years, usually along with rifampin because of synergistic activity. Adverse effects of erythromycin are relatively frequent and include colitis and diarrhea as well as hyperthermia and respiratory distress syndromes. Newer macrolides and a group of macrolide derivatives, the azalide and ketolide drugs, are now available for use in foals. These drugs have fewer adverse effects and are likely more effective than erythromycin in the treatment of foals with pneumonia caused by R equi infection. These drugs also concentrate intracellularly to a greater extent than erythromycin does. The most widely used drugs in this class are clarithromycin and the azalide drug azithromycin.
The pharmacokinetics of azithromycin have been studied in foals. The dosage of azithromycin is 10 mg/kg, administered orally, every 24 hours for 5 days as a loading dose and then every 48 hours. A distinct advantage of azithromycin is its property of concentrating in neutrophils, where it reaches concentrations 200 times those in plasma.
Clarithromycin has also been studied in foals. Oral bioavailability of this drug is also fairly high (57%). Like azithromycin, clarithromycin concentrates intracellularly, particularly in the pulmonary epithelial lining cells and bronchoalveolar cells. The recommended dosage is 7.5 mg/kg, administered orally every 12 hours. Available as a generic formulation, clarithromycin is a very cost-effective long-term treatment. In a study comparing the disposition of erythromycin, azithromycin, and clarithromycin, clarithromycin reached the highest intracellular concentrations in bronchoalveolar cells and pulmonary epithelial lining fluid, followed by azithromycin. In contrast, erythromycin activity in bronchoalveolar lavage fluid was not significantly different from that in serum.
In a retrospective comparison of azithromycin, clarithromycin, and erythromycin for treatment of foals with pneumonia caused by R equi, treatment with a combination of clarithromycin and rifampin was superior to azithromycin-rifampin or erythromycin-rifampin combinations. A tendency toward a higher incidence of diarrhea in foals treated with clarithromycin versus foals treated with azithromycin was reported. In the author’s experience, the risk for diarrhea associated with administration of clarithromycin and azithromycin is similar and is less than that associated with administration of erythromycin.
Additional macrolides have been studied in foals. One of these macrolides is tulathromycin, a triamilide. This drug was administered to foals with pulmonary abscesses (in which the bacterial etiologic agent was not determined) at a dosage of 2.5 mg/kg intramuscularly once weekly, and was compared with orally administered azithromycin. It took significantly longer (53 days vs. 42 days) for the pulmonary abscesses to resolve with tulathromycin. Adverse effects included self-limited diarrhea in 11 of 37 foals, increased rectal temperature in 6 foals, and injection-site swelling in 12 foals. In addition, in vitro activity of tulathromycin against R equi is poor, with achievable plasma and lung concentrations below the MIC values. Therefore tulathromycin cannot be recommended for treatment of R equi infection. Similarly, tilmicosin is poorly active against R equi isolates in vitro and may cause injection site lesions, and thus it cannot be recommended for use against R equi in foals.
Telithromycin is an oral ketolide. Not to be confused with tulathromycin, it has been studied pharmacokinetically. The in vitro activity of telithromycin is significantly higher than that of azithromycin, clarithromycin, and erythromycin against macrolide-susceptible isolates of R equi. A dosage of 15 mg/kg administered daily would be adequate against susceptible isolates. An every-12-hour dosing protocol should be effective against approximately 50% of macrolide-resistant isolates. However, further studies are required to determine the safety and clinical efficacy of repeated doses of telithromycin in foals because the pharmacokinetic study was only a single-dose study.
Gamithromycin is an azalide that is approved for use in cattle for bovine respiratory disease. It has also recently been studied in foals at a dose of 6 mg/kg intramuscularly. It was found to maintain pulmonary and neutrophil concentrations above the MIC(90) of R equi for 7 days. Its in vitro activity against R equi is similar to that of azithromycin or erythromycin. Potential adverse effects include muscle soreness at injection sites as well as diarrhea. This drug is promising for treatment of macrolide-susceptible isolates of R equi but requires further study (multiple dosing).
A few recent findings have been alarming regarding R equi in foals and antimicrobial treatment. The first is the evolution of macrolide- and rifampin-resistant isolates. It is suspected through temporal association that the widespread use of macrolides and rifampin has allowed for development of resistance through selection pressure. Forms of this widespread use include the prophylactic treatment of all foals on farms as neonates, as well as the treatment of subclinical ultrasonographic pulmonary lesions (consistent with abscesses, although not confirmed as R equi), despite studies showing that these subclinical and ultrasound-determined lesions often resolve spontaneously even without treatment. How to optimally handle suspected infections with such subclinical abscesses is the current focus of much study and consideration. The conclusion thus far is that the widespread use of macrolides or rifampin carries risks, and they should not be used as prophylaxis in the neonatal period or in the treatment of subclinical suspected infections.
The second recent finding concerning treatment of R equi infection in foals is that coadministration of rifampin (10 mg/kg, PO, every 12 hours) and clarithromycin for 11 days decreased the bioavailability of clarithromycin by more than 90%. Although the exact mechanisms of this decreased bioavailability are not completely understood, they are possibly the result of an association with induction of intestinal efflux transporters and liver metabolizing enzymes, or inhibition of intestinal uptake transporters. Despite a drop in plasma concentrations below the MIC of R equi, the pulmonary epithelial lining fluid and bronchoalveolar lavage cell intracellular concentrations were still above the necessary MIC (approximately 65% and 80% lower penetration, respectively, than concentrations when clarithromycin was administered alone, however). Although these findings should elicit concern, what they mean with regard to clinical use of clarithromycin-rifampin in foals is unknown. The combination is synergistic in activity against R equi, so the solution is not simply to resort to eliminating rifampin from the regimen. In addition, the dose of rifampin (10 mg/kg) used in these studies is at the high end of the recommended dose range (5 to 10 mg/kg). What effect 5 mg/kg of rifampin would have on the absorption of clarithromycin is unknown. Whether these findings will lead to recommendations for an increase in the dose of clarithromycin is unknown at present. Until further research is available, one strategy for using the combination is to stagger the administration of clarithromycin and rifampin by up to 6 hours.



