CHAPTER 16 Ultrasonographic Imaging of the Gastrointestinal Tract
Ultrasound imaging has become widely accepted as an important diagnostic tool for imaging the gastrointestinal tract in veterinary patients. Initially it was thought that artifacts created by gas and ingested material would significantly limit or exclude the use of ultrasound for examining the gastrointestinal tract. However, experience has shown that these artifacts, although present and at times obstructive, rarely interfere significantly with a thorough ultrasonographic examination. The technology has continued to improve. Higher resolution (12 to 18 MHz and higher) transducers and more affordable equipment—along with the availability of Doppler ultrasound—have made it possible to image the gastrointestinal tract more thoroughly, enhancing the ability to evaluate and understand changes produced by diseases affecting that organ.1
METHOD OF EVALUATION
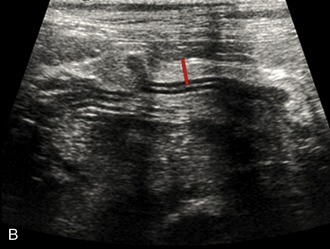
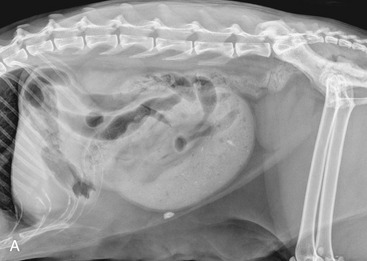
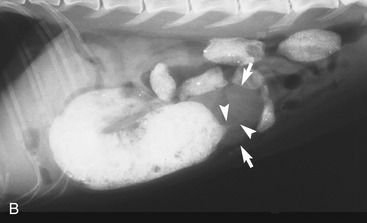
It is preferred, but not always necessary, that the patient be held off food before the ultrasonographic examination. Withholding food for 8 to 12 hours can reduce interference from gas and ingested contents. This is especially helpful when examining the patient for suspected foreign bodies within the intestinal lumen and when evaluating intestinal walls. An enema is not recommended or usually needed because this introduces gas into the colon, which may interfere with the examination. Gas within the lumen often is manifested by reverberation, comet tails, and acoustic shadowing artifacts. A comet-tail artifact is a streaklike or cone-shaped reverberation phenomenon, typically occurring deep to metallic objects. A ring-down artifact is a streaklike reverberation phenomenon, typically occurring deep to a fluid-gas interface. It has the appearance of vertical hyperechoic lines extending from the first encounter with the surface of the air interface to deep within the image. These artifacts can obscure normal or diseased tissue and also can mimic disease. Feces within the colon or barium contrast material anywhere in the intestinal tract can attenuate the sound beam (Figure 16-1). This often obscures the far wall of the intestine and limits the ability to evaluate that section of intestine. Survey radiographs can be helpful in providing an overall view of the abdomen and to confirm or identify sonographic findings.
NORMAL ANATOMY
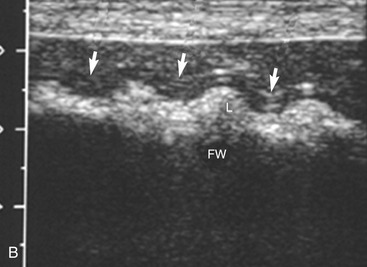
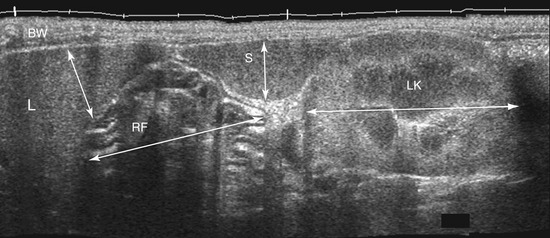
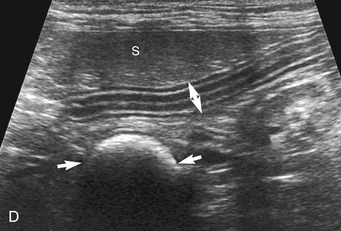
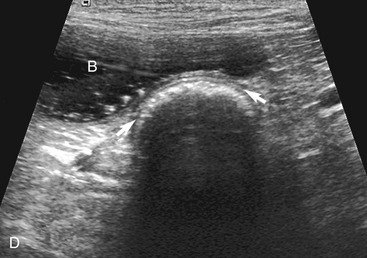
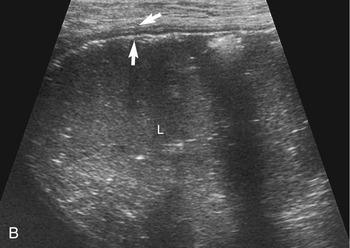
The stomach (the fundus to the pylorus), duodenum, jejunum, ileum, and colon all can be identified separately in the cat. The stomach is located in the left cranial abdomen just caudal to the liver, craniomedial to the head of the spleen, and cranial to the left kidney (Figure 16-2). The wall may be mildly thickened and contain a hyperechoic submucosal layer from adipose tissue, especially in overweight cats (Figure 16-3). The pylorus is located on the midline, as opposed to the right of midline as in dogs. During an abdominal ultrasound examination the stomach usually is empty and typically has a rosette or wagon-wheel appearance. It shows a characteristic pattern with its size, shape, and prominent rugal folds.
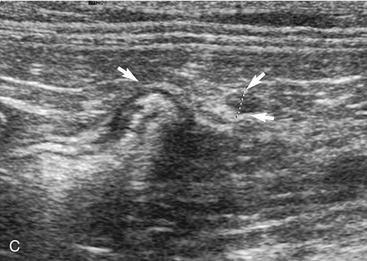
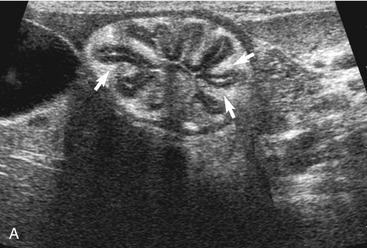
The duodenum courses in a straight superficial path from the pylorus along the right body wall for approximately two-thirds the length of the abdomen. From there it curves medially at the caudal flexure and then courses craniomedially. The sphincter of Oddi, the entrance of the conjoined bile duct and pancreatic duct, is located in the proximal flexure of the duodenum just distal to the pylorus. The pancreatic duct and bile duct join before entering the duodenum at the cistern of Vater. Approximately 90 per cent of cats have only the primary pancreatic papilla; the rest also have a minor duodenal papilla. The Peyer’s patches (lymphoid aggregates), which can be seen on the antimesenteric surface of the duodenum in dogs, are usually not seen in cats. The duodenum, and sometimes parts of the jejunum, can show segmental contractions resulting in a “string of pearls” appearance as opposed to the stripping peristaltic activity seen in dogs (Figure 16-4). The jejunum is identified as a long intestinal segment between the duodenum and the shorter ileum. The ileum is a small section of intestine that can be identified by its characteristic appearance (often contracted, with a “wagon wheel” appearance) and its position at the entrance into the colon. The ileum has a prominent hyperechoic submucosal layer and a corresponding undulating mucosal layer.
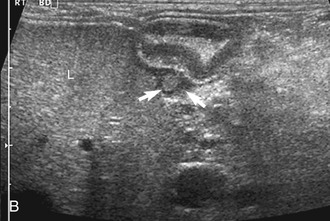
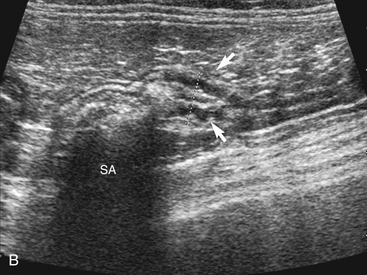
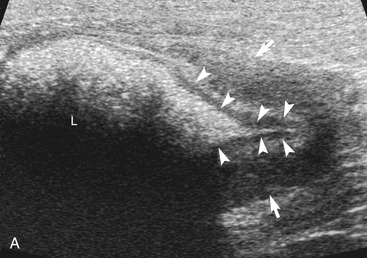
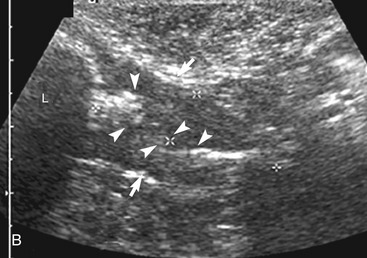
The ileum and/or the cecum can be seen connecting to the larger section of intestine, the colon. The cecum is a cul de sac. It enters the colon adjacent to the ileocolic junction, which is best identified from the right side in the midabdomen. The wall of the tip of the cecum often is thickened and hypoechoic with a reduction in wall layering. Left colic lymph nodes usually are seen in cats near the ileocolic junction, in addition to the mesenteric (jejunal) lymph nodes located at the root of the mesentery. In a recent paper, at least two colic lymph nodes were identified in all of 31 normal cats evaluated.2 The diameter of these lymph nodes ranged from 1.9 to 5.2 mm. In this study, at least one, and usually both, normal medial iliac lymph nodes were found. In interrogations of the intestinal tract, the pericolic and jejunal lymph nodes are especially useful to image and measure because they drain the intestinal tract and can be used as a sentinel reflecting an abnormality within it.
In contrast to the small intestine, the colon is recognized by its larger size, thinner wall, location (extending dorsal to the bladder through the pelvic inlet), and appearance (with sound attenuation and shadowing caused by luminal contents [feces] or reverberations by a gas-distended lumen) (Figure 16-5). The ascending, transverse, and descending colon can be followed. It is easiest to trace the short ascending segment from the right side and the descending segment from the left side of the abdomen. If it is unclear whether a loop of intestine is an abnormal segment of small intestine or colon, it is helpful to follow the loop to see if it courses through the pelvic canal or to trace a segment of bowel known to be colon to the area in question. Small intestine has a thicker wall and does not have the dilated lumen with shadowing contents seen in the colon.
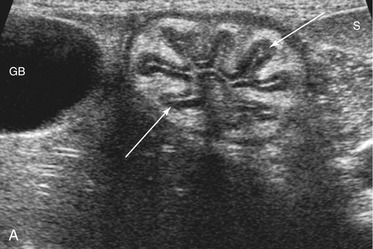
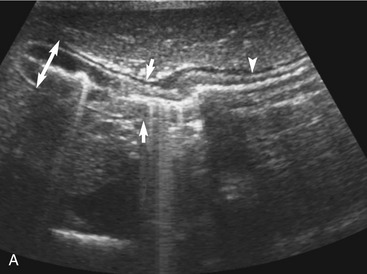
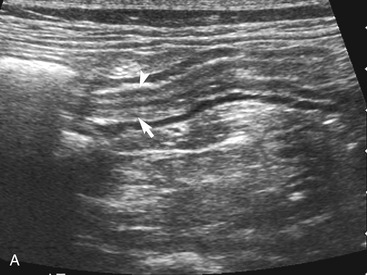
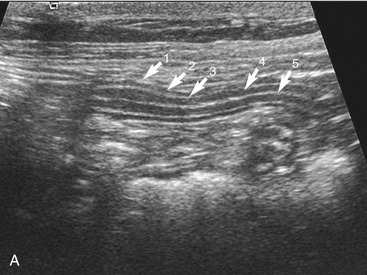
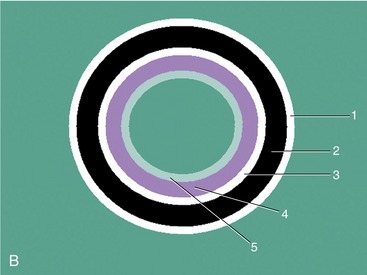
The wall layers of the intestinal tract are similar to the appearance described in human beings and dogs.3 Depending on the section of intestine examined, the wall layer thickness and appearance varies. The wall layers identified sonographically are formed by the interface of specific histological components of the layers of the intestine, which generate different acoustic impedance values between the layers, and by internal reflecting margins within tissue layers. The ultrasound measurements of the apparent layers correspond with the histological measurements but are affected by the axial resolution of the transducer used and the speed of sound in the different tissue layers. The higher the resolution of the transducer, the better the corresponding morphological correlation. The mucosa layer is most apparent in the duodenum and jejunum and less apparent in other segments of the intestine (i.e., colon and ileum), likely because of the change in function and role in the absorptive process. The wall of the intestine includes five layers with an alternating hyperechoic and hypoechoic appearance. The three hyperechoic layers (the S layers) are serosa, submucosa, and the surface of the mucosa; the two hypoechoic layers (the M layers) are the muscularis and the mucosal layers (Figure 16-6).
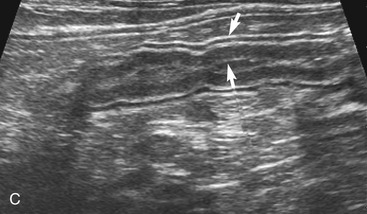
The wall thickness in part is related to the degree of distension of the loop of intestine and gets mildly thinner the more distended it becomes (Figure 16-7). Contraction during normal peristaltic activity does affect the width of the intestinal wall although the width of specific layers can change momentarily with contractions. Because the intestinal tract is dynamic, contractions shorten the loop of intestine in the contracted area and increase the width of the muscular and mucosal layers compared to adjacent dilated segments. It is important to be consistent in placing the cursors for wall measurements. The stomach wall thickness varies from 1.7 to 2.8 mm. It is important to avoid measurement of rugal folds or oblique cuts to prevent erroneously thickened measurements. It is easiest and most accurate to measure the wall when a small amount of fluid is present within the gastric lumen. This may require administering water orally if there is any question about the wall thickness. The thickness of the duodenum and jejunum usually is between 2.3 and 2.8 mm. It is more difficult to measure the wall thickness of the ileum accurately due to its “wagon wheel” appearance. The ileum has a prominent hyperechoic submucosa due to fat and lymphatics, and a hypoechoic muscularis layer. Ileal wall thickness may measure between 2.4 and 2.8 mm. The colonic wall is thinner (usually 1.4 to 2.3 mm), and the wall layers are less distinct.4,5 The far wall of the colon often is obscured by shadowing artifacts due to absorption of sound by fecal material. In general, intestinal wall thickness in cats is fairly consistent at 2.3 to 2.8 mm; it is regarded as abnormally thick when wall thickness is greater than 3 mm or the appearance of individual wall layers changes.
IMAGING OF SPECIFIC GASTROINTESTINAL DISORDERS
LUMINAL CHANGES
Obstruction
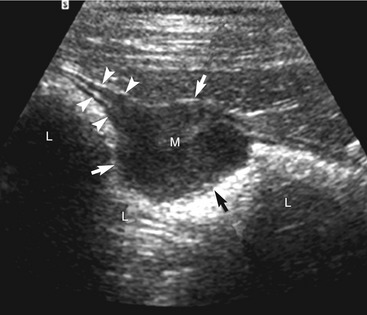
The cause of the obstruction can be intraluminal, mural, or (less often) extramural. In an intraluminal obstruction, the intestinal tract often will be distended with motile, hypoechoic fluid, and possibly gas or food, orad to the point of the obstruction. The fluid may be propelled ineffectively aborad by peristaltic waves and may move back and forth. In a patient with an acute obstruction peristalsis often is increased, but intestinal smooth muscle also may be hypomotile if the obstruction is chronic. If the high obstruction is very proximal within the duodenum, vomiting and thus periodic removal of some of the contents may decrease distension of the lumen. Distension of the intestine due to an obstruction of the distal small intestine may vary depending on the obstruction’s duration and scope (i.e., partial or complete). Segments of small intestine distal to the obstruction usually are of normal size. When a dilated segment of intestine is observed, it is useful to determine the section of intestine involved. To accomplish this, the dilated segment is followed in each direction to the point of the obstruction. By following the segment in each direction, the orad and aborad course of the intestine can be determined. The lumen and wall at the point of the change in diameter of the intestine is examined critically to determine if there is any evidence of a luminal or mural obstruction (Figures 16-8 and 16-9). The lumen is evaluated critically for a focal change in appearance of the contents. This may be manifested by a change in echogenicity, amount of sound attenuated by the contents, or by the shape, size, or margin definition observed. The wall is interrogated for a change in thickness (increased or focally decreased), for a change in echogenicity and visibility of separate wall layers, for gas in the wall, and for plication (Figure 16-10). The adjacent peritoneal cavity also is evaluated for fluid, free air, and hyperechoic omentum, which may be adhered to the serosal wall, consistent with inflammation and possibly intestinal leakage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree