Chapter 16 Ultrasonographic Evaluation of the Equine Limb
Technique
Diagnostic ultrasonography was introduced in the early 1980s as a practical imaging modality to evaluate soft tissue injuries of the equine limb.1 It continues to be used extensively for evaluation of tendonous and ligamentous structures to identify, confirm, and monitor soft tissue injury (Box 16-1).2-8 Ultrasonography is now the imaging modality of choice for most soft tissue evaluation, but in the digit magnetic resonance imaging (MRI), if available, is most useful to evaluate soft tissues. However, it is user specific, and diagnostic information relies heavily on adequate equipment, limb preparation, and the scanning skills of the ultrasonographer. This chapter describes terminology and techniques and provides advice about interpretation based on our collective experiences. We describe our systematic approach, which includes both qualitative and quantitative ultrasonographic analysis of distal limb injuries. The resulting data can be used to categorize the severity of the injury and compare with subsequent examinations. The technology is still developing, and changes will evolve to improve the clinician’s ability to identify and substantiate clinical findings, leading to improved management of soft tissue injury in the horse.
BOX 16-1 Indications for Ultrasonographic Evaluation of Limbs
Medical Records
Thermal Print Storage Envelope
We have developed an alphanumerical system for ranking exercise levels (Box 16-2). The current exercise level may have an important impact on diagnosis, treatment, future exercise control, and prognosis. For instance, if a horse at pasture injured a SDFT, advising pasture rest would be contraindicated. A chart for ranking exercise levels can be duplicated easily, laminated, and taped to equipment for easy reference.
BOX 16-2 Exercise Level Grading Scale
| Exercise Level | Type of Exercise Allowed |
|---|---|
| 0 | Complete stall rest. |
| 1A | Handwalk for 15 to 30 minutes once a day. |
| 1B | Handwalk ≥30 minutes a day or walk on a mechanical walker. We believe horses usually are more active walking on a mechanical walker than in hand. |
| 2A | Thoroughbred (TB) and Standardbred (STB) racehorse, event horse (EV), and sports horse (SH): exercise level 1A/B plus trotting in hand for 5 to 10 minutes a day. |
| 2B | TB racehorse, SH, and EV: trot under saddle 10 to 15 minutes once a day or swim. |
| STB racehorses: walk only in the bike or swim. | |
| This level also includes 10 to 15 minutes of trotting on a treadmill. | |
| 3A | Small paddock turnout for all horses. Small paddock implies small enough not to be able to work up to a sustained canter or gallop. |
| 3B | Large paddock turnout for all horses. |
| 4A | TB and SH: 15 to 20 minutes a day of walk, trot, and canter under saddle, 3 days per week, plus any of the above levels. This level does not apply to most STBs. |
| 4B | TB: 20 to 30 minutes a day of walk, trot, and canter under saddle, 3 to 4 days a week. |
| TB racehorse: “ponying” (being led from another horse) on the racetrack. This level does not apply to most STBs. | |
| 5 | TB racehorse and EV: all of the above plus normal galloping. |
| STB racehorse: all of the above plus jogging. | |
| SH: all of the above plus normal arena flat work with limited low fence jumping where applicable. | |
| Dressage horses: normal work minus lateral movements and special gaits. | |
| 6 | TB racehorse: all of the above plus faster gallops. |
| EV: all of the above plus jumping. | |
| STB racehorse: all of the above plus training miles ≥2 : 10. | |
| SH: all of the above plus unlimited low fence jumping where applicable. | |
| Dressage: all of the above plus lateral movements and special gaits. | |
| Contest horses (reiners, cutters, and so on): all of the above plus practicing specific turns and movements. | |
| 7 | In essence, this is the maximal work level for any type of athletic horse. |
| TB and EV: racing, fast works, and competing. | |
| STB racehorse: training miles <2 : 10 and racing. | |
| SH: showing, jumping, hunting, and so on. | |
| Dressage and contest horses: competing. |
Clinical findings may be recorded concisely as follows:
| Leg | Which Limb |
| S | Subcutaneous swelling on a scale of 0 to 5. This does not refer to tendonous or ligamentous enlargement. |
| L | Lameness on a scale of 0 to 5. |
| T | Thickening of a tendon or ligament on a scale of 0 to 5. This is independent of subcutaneous swelling. |
| Sen | Response to digital palpation of a tendon or ligament on a scale of 0 to 5. |
| H | Heat or skin temperature on a scale of 0 to 5. |
| TS | Distention of the digital flexor tendon sheath (DFTS) (tenosynovitis) on a scale of 0 to 5. |
| AS | Fetlock sinking (hyperextension of the metacarpophalangeal joint), either at rest or during movement, on a scale of 0 to 5. |
| Qualitative diagnosis | Comments on preliminary qualitative ultrasonographic interpretation. |
| New | Whether this is the first time this horse has been examined ultrasonographically for this complaint. |
| Re-Ck | A recheck of a previous injury. |
| Normal leg | This indicates which is the clinically normal limb, which may not be normal ultrasonographically. It is strongly recommended that both limbs be examined routinely. |
| Both abn | Both limbs are clinically abnormal. |
Exercise Levels
One of the basic concepts in the management of horses with tendon and ligament injuries is to relate increasing levels of exercise to increases in tendon and ligament loading. For instance, walking a Thoroughbred (TB) racehorse in hand results in far less SDFT loading (stretching) than racing at 35 mph. Understanding the current exercise level is important in interpretation of ultrasonographic information and advising management programs for controlled exercise during rehabilitation from an injury (see Box 16-2).
Artifacts
Operator Errors
Inadequate Skin Preparation
Excessively long or dirty hair and unclean skin attenuate the ultrasound beam and produce image artifacts. Improper scanhead coupling to the skin caused by a lack of gel or the presence of scabs or scurf is common (Figure 16-1). Artifacts created by improper skin preparation result from poor contact. Images may be dark even though gain settings may be set at the highest limits. The tendons do not have a fine texture and are more grainy or mottled than normal. Tendon margins may not be seen, and reverberation artifacts caused by trapped air within the hair may appear within images, especially if standoff pads are used. Hypoechogenic streaks may be present in the images.
Ultrasound Beam Angle
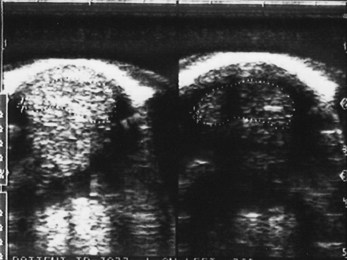
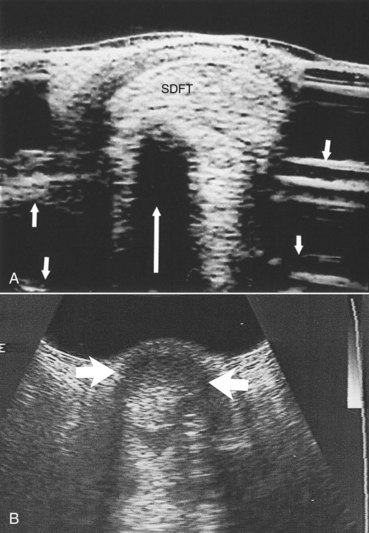
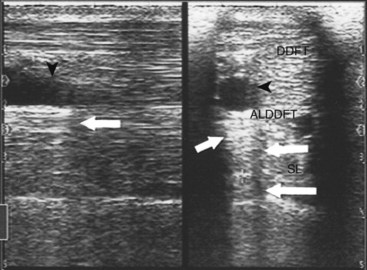
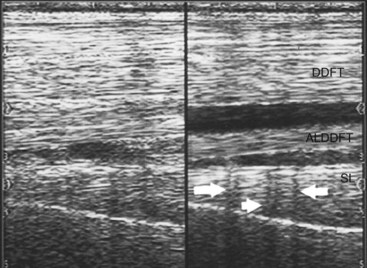
Reflection of the ultrasound beam is dependent on the sound-interface and tissue-interface geometry. Ideally the ultrasound beam should strike tissue interfaces at 90 degrees to produce the best echo reflection back to the transducer crystals, which also act as receivers. If the beam strikes a tissue interface at a smaller angle, a portion of the ultrasound is reflected away from the primary beam direction, and the interface is not seen as well or at all. This is especially important in the evaluation of tendons and ligaments in the metacarpal and metatarsal regions because the fibers usually are parallel to the skin. If the ultrasound sound beam is not perpendicular to a tendon in a transverse image, information is lost, and hypoechogenic areas, which mimic lesions, are created (Figure 16-2). The problem is not seen in longitudinal images of the metacarpal or metatarsal region obtained using a linear array transducer because its surface is parallel to the fibers. However, with convex array and sector transducers that have divergent ultrasound beams, there are only small areas within longitudinal tendon fiber images in which valid information is found (Figure 16-3). In the divergent area of the beam, sound is reflected away from the beam path and is lost to the image. It is important not to confuse these areas with pathological conditions of the tendon or ligament. The ultrasound beam must be positioned parallel to the tendon fibers. Normal tendon or ligament fibers should be seen as continuous linear echogenic structures across the image (Figure 16-4). If the transducer is turned slightly, the fibers appear as short linear segments as the beam cuts obliquely across the longitudinal axis. Because fiber alignment is an important criterion to assess in diagnosis and rehabilitation of tendon and ligament injuries, care must be taken to not create this artifact.
Improper Gain Settings
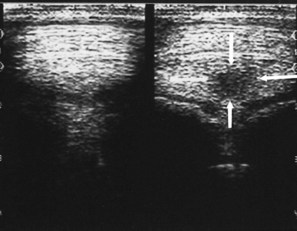
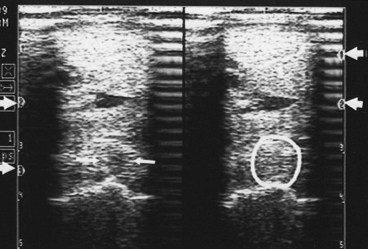
Ultrasound machines have gain settings referred to as overall, near, and far gain. As ultrasound penetrates normal soft tissues, it is attenuated at the rate of 1 dB/cm of tissue thickness per megahertz. Obesity and dehydration cause greater attenuation and can limit penetration. Because energy is lost from the ultrasound beam as it passes into the tissues, the instrument gain must be increased to allow echo detection from the deeper tissue depths. Overall gain changes the brightness and darkness over the entire image. The near-gain adjustment affects the echoes closest to the transducer. Increasing the near gain brightens the echoes, and decreasing it darkens them. The far gain affects the deeper aspect of the image. Some machines display a time-gain compensation curve at the side of the screen. Adjustment of the gain settings should produce an image in which the gray scale is similar over the entire image. Bright whites in the near field should prompt decreasing the near gain; conversely, if echoes are difficult to see, increase in the gain is necessary (Figure 16-5). Improper near-gain settings are the most common error in settings. Visibility of far-field echoes is enhanced by far-gain increases.
Improper Focal Zone Use
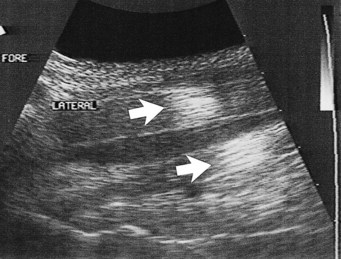
Before the advent of moveable and multiple focal zones, transducers had fixed focal points and well-defined focal zones. The ultrasound beam was narrowest in the focal zone in which the lateral resolution produced the best image quality. If the tissues of interest were outside the focal zone, image quality was not ideal. Use of transducers with appropriate focal zones became necessary. If a sector transducer is used to image the SDFT, a standoff pad is necessary to place the tendon in the focal zone. Variable focal zones have eliminated this problem and allow focal zone placement throughout the image depth. Modern linear array and convex array transducers have multiple and moveable focal zones. However, they need to be set in the proper locations to investigate the areas of suspected abnormality. If the focal zones are not placed properly, clinically significant tendon and ligament fiber damage can be overlooked (Figure 16-6).
Recording Images
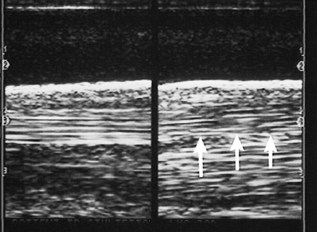
Thermal printers were the most popular method for recording ultrasonographic images, and some are still in use. Brightness may be set too high, causing echoes to have no differentiating characteristics (Figure 16-7), or set too low, causing the image to be too dark (Figure 16-8). The same problem occurs with contrast settings; too high a setting causes excessive contrast, and too low a setting causes the image to be washed out and too dark.
Standoff Pads
Standoff pads are necessary when scanning structures near the skin, such as the SDFT and SL branches. Artifacts are produced each time they are used and can compromise image quality. It is important to recognize them. Transducers with built-in fluid standoff devices are no different. The artifacts are caused by reverberation within the standoff material and occur at a depth equaling the pad thickness (see Figure 16-5), which may obscure detail. For instance, examination of SL branches with a standoff pad can place artifacts in or near the axial border of the SL. This technique can obscure small axial border tears that are fairly common in horses. If the standoff pad thickness produces interfering artifacts, the SL branch should be examined with and without a standoff pad.
Ultrasound Tissue Interaction Artifacts (Horse-Produced Artifacts)
Skin Surface Contact
Debris and air trapped in the hair create large acoustic impedance differences and do not allow adequate sound penetration (see Figure 16-1).
Acoustic Enhancement
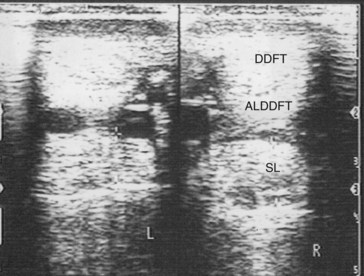
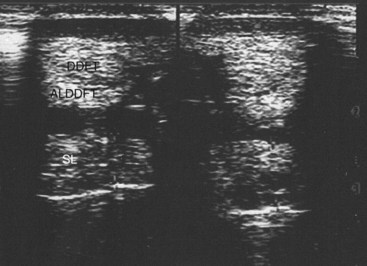
Whenever ultrasound passes through nonattenuating tissues, such as fluid with few or no interfaces, a slight increase in ultrasound intensity occurs in addition to less attenuation. The adjacent tissues attenuate the ultrasound normally. This causes brighter (enhanced) echoes deep to the less attenuating tissues. A good example is the enhancement seen deep to the metacarpal and metatarsal blood vessels. The SL has brighter echoes deep to the blood vessels, whereas between the blood vessels it is less echogenic (darker); it is tempting to interpret such results as abnormal (Figure 16-9). This is an inherent, tissue-produced artifact that can lead to interpretation errors but may help identify less attenuating areas within tissues such as muscle.
Refractive Scattering
If the ultrasound beam is not perpendicular to a tissue interface, hypoechogenic artifacts are caused by refraction. These artifacts can be a major problem in assessing the origin of the SL because of the anastomotic veins between it and the ALDDFT (Figure 16-10). The curved blood vessel walls create hypoechogenic lines that extend deep to the vessel walls in transverse images (see Figure 16-9). This is called refractive scattering and should not be misinterpreted as a lesion.
Acoustic Shadowing
A high acoustic impedance difference blocks the ultrasound beam and causes an anechogenic shadow deep to the reflecting interface. Bone and other mineralized tissue are much denser than soft tissues, and the sound propagation velocity is much faster. This creates an impenetrable acoustic barrier, and characteristically the reflector surface has bright echoes and the deeper tissues cannot be seen. These artifacts are useful because they identify soft tissue mineralization (see Figure 16-5). Bone surfaces are easily recognized because of shadowing. Dense scar tissue may create incomplete shadowing.
Reverberation
Reflection of ultrasound back and forth to the transducer from high acoustic impedance interfaces produces reverberation artifacts. The classic example is an air-filled lung surface that produces characteristic concentric reverberation echoes. Reverberation artifacts are not a major problem in tendon and ligament scanning; however, they can be important in certain circumstances. Gas production by anaerobic bacteria and air accidentally injected during diagnostic analgesia are two examples in which reverberation can be found in soft tissues. Standoff pads also create reverberation artifacts (see Figure 16-5).
Terminology and Quantitative Measurements
The basis of diagnosis is to determine the morphological variation from normal, which is not always easy. The goal is to determine the size, shape, echogenicity, fiber pattern, and surrounding inflammatory reaction of any structure. These findings should be considered carefully in conjunction with clinical impressions and the current athletic use of the horse. Box 16-3 lists parameters used for characterizing tendon and ligament lesions.15
Echogenicity
Echogenicity refers to the whiteness or brightness of a structure. Each tendon and ligament has a characteristic echogenic pattern at specific anatomical sites. Lesions vary in echogenicity depending on morphological consistency at the time of the examination. A scoring system can be used to improve objectivity when assessing the severity of an injury or the response to therapy (Box 16-4). Such a system may improve case management and illustrate to the client the changes in echogenicity that correlate with repair of an injury.