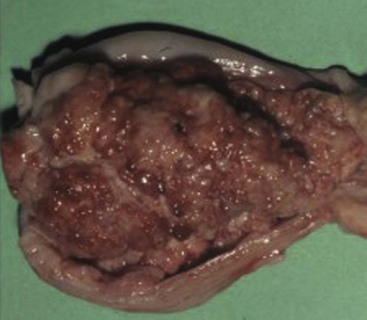
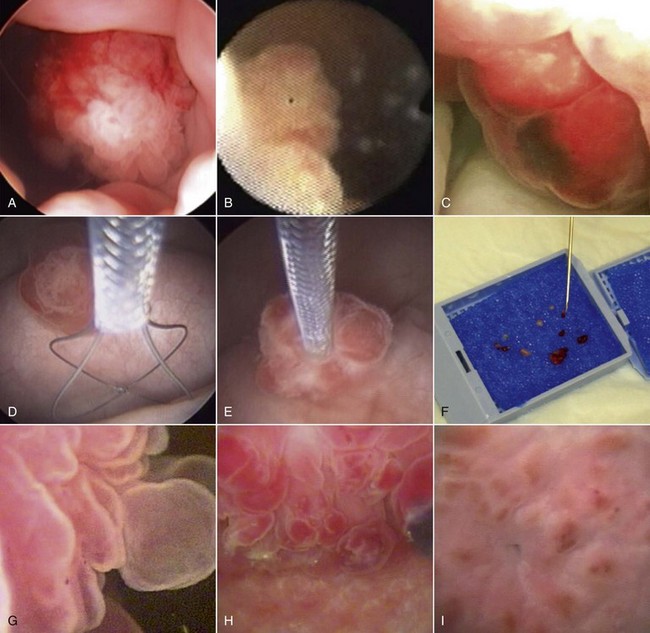
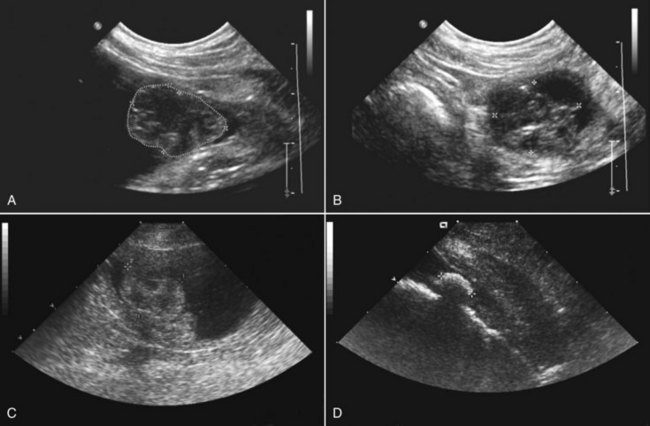
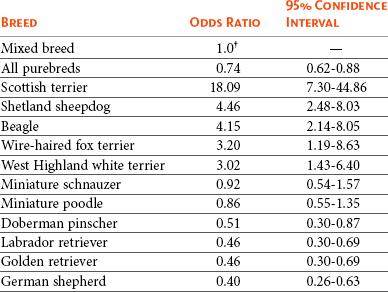
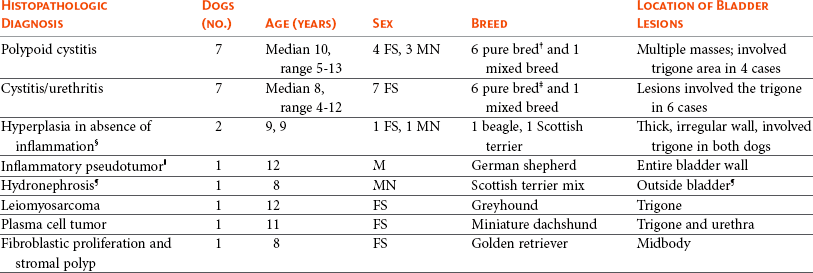
29 Urinary bladder cancer accounts for approximately 2% of all reported malignancies in the dog.1–4 With more than 70 million dogs in the United States, even uncommon forms of cancer affect thousands of dogs each year.2,3 The hospital prevalence or proportionate morbidity of bladder cancer at university-based veterinary hospitals is increasing.2 Invasive transitional cell carcinoma (TCC) is the most common form of canine urinary bladder cancer.1–4 Most TCCs are intermediate- to high-grade papillary infiltrative tumors.2–4 Other types of bladder tumors reported less frequently include squamous cell carcinoma, adenocarcinoma, undifferentiated carcinoma, rhabdomyosarcoma, lymphoma, hemangiosarcoma, fibroma, and other mesenchymal tumors.4–10 TCC is most often located in the trigone region of the bladder. Papillary lesions and a thickened bladder wall (Figure 29-1) are common features and can lead to partial or complete urinary tract obstruction. In a series of 102 dogs with TCC of the bladder, the cancer also involved the urethra in 56% of dogs and involved the prostate in 29% of male dogs.2 Nodal and distant metastases were present in 16% and 14% of dogs, respectively, at diagnosis.2 At the time of death, distant metastases were detected in 50% of the dogs.2 Following World Health Organization (WHO) criteria for staging canine bladder tumors11 (Box 29-1), 78% of dogs had T2 tumors and 20% had T3 tumors.2 The etiology of canine bladder cancer is multifactorial. Risk factors include exposure to older-generation flea control products and lawn chemicals, obesity, possibly cyclophosphamide exposure, female sex, and a very strong breed-associated risk (Table 29-1).1,2,12–15 The female : male ratio of dogs with TCC has been reported to range from 1.71 : 1 to 1.95 : 1.1–3 TCC risk is higher in neutered dogs than in intact dogs of both sexes, although the reason for this has not been determined.2,13 Table 29-1 Breed and Risk of Transitional Cell Carcinoma (TCC) in Pet Dogs* *This table represents a summary of data from 1290 dogs with TCC and 1290 institution and age-matched control dogs without TCC in the Veterinary Medical Data Base. Modified from previous report by Knapp DW, Glickman NW, DeNicola DB, et al: Naturally-occurring canine transitional cell carcinoma of the urinary bladder: a relevant model of human invasive bladder cancer, Urol Oncol 5:47–59, 2000. Two case control studies have implicated chemical exposure in TCC risk in dogs. In a case control study of dogs of several breeds, an association between TCC and exposure to topical flea and tick dips was noted.12 In the highest risk group (overweight female dogs), the risk of TCC was 28 times that of normal-weight male dogs not exposed to the insecticides.12 The authors speculated that the “inert” ingredients (solvents and petroleum distillates), which accounted for more than 95% of the product, were the probable carcinogens. Newer, spot-on type flea control products appear safer. In a case control study in Scottish terriers, spot-on products containing fipronil were not associated with increased risk of TCC.14 In a case control study in Scottish terriers (STs), exposure to lawn herbicides and pesticides was compared between STs with TCC (n = 83) and a control group of STs (n = 83, ≥6 years of age, no history of urinary tract disease in the previous 2 years).15 TCC risk was significantly higher in STs that had been exposed to lawn herbicides alone (odds ratio [OR], 3.62; 95% confidence interval [CI] 1.17 to 11.19; p <0.03) or herbicides and insecticides (OR, 7.19; 95% CI 2.15 to 24.07; p <0.001) than in dogs not exposed.15 A positive finding in the STs study was that dogs that ate vegetables at least three times a week, along with their normal diet, had a reduced risk of TCC (OR, 0.30; 95% CI 0.01 to 0.97; p <0.001).16 The specific type of vegetable with the most benefit could not be determined, but carrots, given as treats, were the most frequently fed vegetable in the study. Many conditions can mimic TCC in regards to clinical signs, abnormal epithelial cells in urine, and mass lesions within the urinary tract (Figures 29-2 and 29-3; Table 29-2). Differential diagnoses include other neoplasia, chronic cystitis, polypoid cystitis, fibroepithelial polyps, granulomatous cystitis/urethritis, gossypiboma, calculi, and inflammatory pseudotumor.4–10,17–21 It is important to distinguish non-TCC conditions from TCC because the treatment and prognosis differ considerably and depend on the condition present. Table 29-2 Characteristics of 21 Dogs Presented to the Purdue University Veterinary Teaching Hospital (PUVTH) in Recent Years With Bladder/Urethral Masses Caused by Conditions Other Than Transitional Cell Carcinoma (TCC)* FS, Female spayed; MN, male neutered; M, male. *Excludes dogs with prostatic carcinoma or other prostatic disease and dogs with cystic calculi. †One each of the following: Australian shepherd, Jack Russell terrier, Bichon Frise, Swiss mountain dog, golden retriever, Rhodesian ridgeback. ‡One each of the following: German shepherd, Great Dane, Cocker spaniel, English Springer spaniel, West Highland white terrier, Chesapeake Bay retriever. §One dog had surgical biopsies. The other dog had cystoscopic biopsies and no evidence of progression to TCC >2 years later. ¶The dog was referred to the PUVTH for inappropriate urination and abdominal mass. Based on signalment and history, TCC was included on the differential list. Ultrasonography and computed tomography (CT) demonstrated a large cystic mass associated with the right kidney. A 15 × 10 × 10 cm mass containing 500 mL of fluid was removed surgically. Histopathology of the mass revealed renal atrophy, fibrosis, and moderate hydronephrosis, most likely due to a urinary obstruction event. No cause for urinary obstruction was found, and the dog was normal at last follow-up 7 months post op. A diagnosis of TCC requires histopathologic confirmation. Although neoplastic cells may be present in the urine of 30% of dogs with TCC,4 neoplastic cells often are indistinguishable from reactive epithelial cells associated with inflammation. Urine antigen tests for TCC have been found to be sensitive,22 but a high number of false-positive results limits the value of these tests. A bladder or urethral mass may raise suspicion for TCC, but as discussed previously, other conditions can cause bladder and urethral masses (see Figures 29-2 and 29-3 and Table 29-2). Therefore histopathologic examination of the abnormal tissues is essential to determine whether TCC is present. In addition, different pathologic types of TCC can be identified with histopathology.4,21 Methods for obtaining tissue for histopathologic diagnosis include cystotomy, cystoscopy (see Figure 29-2), and traumatic catheterization.2,23–25 Cystoscopy provides the opportunity to visually inspect the urethra and bladder and to obtain biopsies in a noninvasive method. With the small size of cystoscopic biopsies, the operator must be diligent to collect sufficient tissue for diagnosis. Placing tissue samples in a histology cassette prior to processing helps prevent loss of small samples (see Figure 29-2, F). In a recent report of 92 dogs, diagnostic samples were obtained by cystoscopy in 96% of female dogs and 65% of male dogs that ultimately had hisotopathologically diagnosed TCC.25 The more recent use of a wire basket designed to capture stones during cystoscopy (see Figure 29-2, D and E) allows collection of larger samples and is expected to increase the yield of diagnostic biopsy samples. Traumatic catheterization to collect tissues for diagnosis can also be performed, although samples collected by this method are usually small and the diagnostic quality varies considerably from case to case. Percutaneous biopsy methods can lead to tumor seeding and are best avoided.26,27 In poorly differentiated carcinomas, immunohistochemistry for uroplakin III (UPIII) can be helpful in distinguishing TCC from other carcinomas. UPIII, a transmembrane protein expressed in superficial transitional epithelial cells in the urinary tract, is expressed in more than 90% of canine TCC and has been considered a specific marker for TCC.28 Recently, UPIII expression has also been reported in canine prostate cancer,29 although it is not known if the immunoreactive cells originated from the transitional epithelium of the prostatic ducts or elsewhere in the gland. Common clinical signs in dogs with TCC include hematuria, dysuria, pollakiuria, and, less commonly, lameness caused by bone metastasis or hypertrophic osteopathy.1 Urinary tract signs may be present for weeks to months and may resolve temporarily with antibiotic therapy. In dogs with confirmed or suspected TCC, evaluation should include an assessment of overall health (complete blood count [CBC], serum biochemistry profile, urinalysis, ± urine culture) and staging of the cancer (thoracic radiography, abdominal ultrasonography, and urinary tract imaging). To avoid the risk of seeding TCC through cystocentesis, urine may be collected by free catch or catheterization. If a catheter is to be passed, care must be taken to avoid penetrating the diseased bladder or urethral wall. Common sites of metastases detected with thoracic radiography and abdominal ultrasonography include lymph nodes, liver, and lungs, although metastases can occur in other areas as well.1–3 TCC infrequently metastasizes to bone. Side-by-side comparison of radiographs and a nuclear bone scan may help detect possible bone metastases in dogs with unexplained lameness or bone pain. Urinary tract imaging is used to assess the TCC location for potential surgical intervention and to map and measure TCC masses in order to subsequently determine response to medical therapy. Mapping TCC in the bladder, proximal urethra, and prostate can be accomplished by cystosonography (see Figure 29-3), cystography, or computed tomography (CT).30,31 Regardless of the imaging technique used, to accurately track response to therapy, it is important to follow a consistent protocol from visit to visit for bladder distension and patient positioning. In addition, when using cystosonography to monitor therapy response, it is critical to have the same operator perform the examinations over multiple visits. Surgery and Nonsurgical Procedures Complete surgical excision of TCC is not usually possible because of the typical trigonal location, urethral involvement, and metastases in some cases. Although techniques for trigone resection32 or cystectomy33,34 and the use of grafting materials to replace bladder tissues35,36 have been reported in the dog, these approaches are associated with substantial morbidity and expense and in most cases are not feasible. In addition, many dogs develop multifocal TCC in the bladder, consistent with the “field effect” proposed in humans in which the entire bladder lining is thought to undergo malignant change in response to carcinogens in the urine. In a series of 67 dogs with TCC that underwent surgery for biopsy or for therapeutic intent, complete surgical excision of the tumor with tumor-free margins was possible in only two dogs.2 One of the two dogs had a relapse in the bladder 8 months later, and the second dog developed metastatic disease. Although surgery is rarely curative in dogs with TCC, surgery can be important in restoring or maintaining urine flow. Ureteral stents, when indicated, have traditionally been placed surgically, although less invasive techniques to place stents have recently been described.37 Previously described ureterocolonic anastomosis is not recommended due to the high complication rate and limited survival.38 Prepubic cystostomy catheters that bypass urethral obstruction can be very effective.39,40 More recently, the placement of urethral stents has gained favor over cystotomy tubes in some cases because external tubes are avoided and the pet owner does not have to drain the bladder.41 The survival following stent placement can vary considerably from dog to dog. In preliminary findings from Purdue University, survival following stent placement in dogs ranged from a few days to a year.42 Urethral stents can be placed nonsurgically with fluoroscopic guidance. Although there has been interest in the potential use of laser ablation of tumor tissue to relieve urethral obstruction, in published work to date this has not been successful due to advanced local disease, complications of the procedure, and local disease recurrence.43 One of the challenges in transurethral resection is the difficulty in judging the deeper tumor margin and the risk of cutting too deep and perforating the urinary tract.
Tumors of the Urinary System
Canine Urinary Bladder Tumors
Etiology and Prevention
Diagnosis and Differential Diagnoses
 The dog was presented for urinary obstruction and bladder mass. Prior to presentation at the PUVTH, surgery had been performed to remove a testicle from the abdomen. The testicle and spermatic cord were noted to be wrapped around the urethra. The mesenchymal changes were thought to be due to the bladder response to chronic urethral obstruction.
The dog was presented for urinary obstruction and bladder mass. Prior to presentation at the PUVTH, surgery had been performed to remove a testicle from the abdomen. The testicle and spermatic cord were noted to be wrapped around the urethra. The mesenchymal changes were thought to be due to the bladder response to chronic urethral obstruction.
Presentation and Clinical Staging
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Tumors of the Urinary System