Chapter 23. Tumors of the Skin, Subcutis, and Other Soft Tissues
SECTION A Skin Tumors
Nicole Northrup and Tracy Gieger
KEY POINTS
• Owners should be instructed to perform monthly examinations of their pets to look for skin masses.
• All cutaneous and subcutaneous masses should be aspirated for cytologic evaluation.
• If cytology of a skin mass does not yield a diagnosis, biopsy is indicated.
This section discusses management of skin and subcutaneous tumors of cats and dogs. The reader is directed to discussions on mast cell tumors (MCTs) (see Section D ), soft-tissue sarcomas (STSs; see Section E ), and lymphoma ( Chapter 25, Section A ) for information about these tumors.
Incidence
Tumors of the skin and subcutaneous tissues are common in veterinary patients. 1-5 They are classified as epithelial, mesenchymal, melanocytic, or round cell (discrete cell) in origin. Most are primary tumors, but metastases and cutaneous paraneoplastic conditions are possible. In cats, skin tumors are more likely to be malignant. 4,5 The most common cutaneous tumors of cats and dogs are listed in TABLE 23-1 and TABLE 23-2 .
| ∗ Based on biopsy and necropsy specimens from 340 cats submitted to the University of Missouri Veterinary Medical Diagnostic Laboratory 4 and 3260 surgical biopsy specimens from skin lesions of cats submitted to the Laboratory of Pathology at the University of Pennsylvania, School of Veterinary Medicine. 5 | ||
| Tumor | Frequency (Miller) | Frequency (Goldschmidt) |
|---|---|---|
Basal cell tumor ∗ Mast cell tumor Squamous cell carcinoma Fibrosarcoma Sebaceous adenoma Fibroma Apocrine adenocarcinoma Apocrine adenoma Malignant fibrous histiocytoma Hemangiosarcoma Hemangioma Melanocytic tumors Lymphoma Trichoepithelioma Fibropapilloma Lipoma Undifferentiated carcinoma Undifferentiated sarcoma Basal cell carcinoma | 26% 21% 15% 15% 4% 3% 3% 3% 3% 2% 1% <1% <1% <1% <1% <1% <1% <1% Not reported | 15% 13% 10% 17% 2% Not reported 3% 5% 1% 3% 2% 1% 3% <1% Not reported 6% Not reported Not reported 1% |
| ∗ Based on 29,510 surgical biopsy specimens from skin lesions of dogs submitted to the Laboratory of Pathology at the University of Pennsylvania, School of Veterinary Medicine. 5 | |
| Tumor Type | Frequency (Goldschmidt) |
|---|---|
Histiocytoma Perianal adenoma Mast cell tumor Lipoma Hemangiopericytoma Sebaceous adenoma Trichoepithelioma Hemangioma Melanocytic tumor Basal cell tumor ∗ Fibroma Fibrosarcoma Squamous cell carcinoma Intracutaneous cornifying epithelioma Plasmacytoma Pilomatricoma Lymphoma Hemangiosarcoma Papilloma Basal cell carcinoma Apocrine gland carcinoma | 12% 12% 10% 8% 7% 4% 4% 4% 4% 4% 2% 2% 2% 2% 1% 1% 1% 1% <1% <1% <1% |
Diagnosis and Staging
Cutaneous tumors are readily identified by inspection and palpation of the skin. In patients with thick haircoats, effort should be made to palpate the skin and part the hair to identify any masses. It is important to examine areas less visible to owners, including lips, ears, ventrum, paws, and the axillary, ventral cervical, inguinal, perianal, and genital regions. Clinicians should look for preneoplastic lesions including non-healing scabs, discolorations, and skin texture changes in lightly pigmented, thin-haired areas susceptible to solar damage. Skin lesions should be documented in the medical record on a body map (included on the website, www.smallanimaloncology.com ) or with a digital photograph. Masses should be measured with calipers, and fine-needle aspirates should be obtained for cytology. Biopsy and histology are indicated for masses that are growing, changing in appearance, or irritating the patient and when cytology does not provide a definitive diagnosis.
The decision of whether incisional or excisional biopsy is appropriate (see Chapter 6 ) is based on the size, location, and suspected diagnosis of the mass. Histology provides important information including diagnosis, assessment of completeness of excision with margin measurements, mitotic index, histologic grade, and identification of vascular/lymphatic invasion. For some tumors, immunohistochemistry or special stains may be required to determine the diagnosis. In addition, assessment of proliferation indices is a valuable prognostic indicator for some tumors.
Therapy
For most skin tumors, surgical excision is the treatment of choice. The extent of surgery required depends on the tumor type (see Chapter 14 ). Tissues shrink and shift in formalin, 6 so small samples should be placed in cassettes; for larger excisions, the subcutaneous tissue should be sutured to the skin. A complete history and description of the mass should be provided for the pathologist. Any resected tissues should be submitted in entirety for histology, even if a benign diagnosis is expected, and the surgical margins should be marked with India ink or the multi-colored Davidson Marking System (Davidson Marking System, Bradley Products, Inc., Bloomington, MN).
Primary re-excision of the scar or radiation therapy should be considered for incompletely excised skin tumors. Re-excision is indicated when a relatively conservative surgery was performed to remove a malignant tumor in an area where there is adequate tissue to allow resection of the scar with 2- to 3-cm lateral margins and an intact tissue plane at the deep margin. For tumors not amenable to complete excision, radiation therapy is appropriate for local control. Chemotherapy is indicated for some malignant skin tumors based on diagnosis, grade, and the presence of vascular/lymphatic invasion on histology or nodal metastasis. Treatment protocols depend on the patient’s overall health and diagnosis. Consultation with an oncologist is recommended.
EPITHELIAL TUMORS
Epidermal Tumors
Squamous Cell Carcinoma (SCC), Multi-Centric Squamous Cell Carcinoma in situ (MSCCIS; Bowenoid in situ Carcinoma)
Incidence/Etiology and Risk Factors
Cutaneous SCC is common in cats and dogs 4,5 and accounts for 25% to 52% of digital tumors in dogs. 10,11 SCC is most commonly seen in sparsely haired, poorly pigmented areas of the epidermis. 12-15 In these cases, it is considered to be solar induced and is often preceded by evidence of solar-induced inflammation (actinic keratosis). SCC may develop consequent to chronic inflammation as a result of immune-mediated, infectious (e.g., viral papillomatosis in dogs 16,17 ) causes, and burns. Multi-centric SCC in situ of cats is not solar induced; rather, a viral etiology is suspected. 18,19
Nail bed SCC occurs most commonly in older (median age, 10 years) male dogs > 30 kg and is seen most commonly on the forelimbs. 10,11 Giant schnauzers and standard poodles are breeds at risk for development of multiple digital SCC. 20,21
Clinical Features
SCC appears as solitary or multiple erosive, proliferative, or nodular dermal lesions that may be accompanied by erythema, scale, and concurrent solar-induced tumors (e.g., dermal hemangioma and hemangiosarcoma [HSA]; Figure 23-1 ). In cats, SCC is most commonly seen in white cats on poorly haired areas of the ear tips, preauricular areas, nasal planum, and periocular regions. 12,13,15 Early lesions are often mistaken for scratches but do not heal.
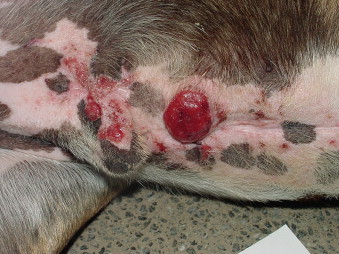 |
| FIGURE 23-1 Solar-induced squamous cell carcinomas are frequently seen on the ventral abdomen in non-pigmented skin of dogs with sparse haircoats and a history of sunbathing. |
Multi-centric SCC in situ (MSCCIS) is seen in cats of all colors. Lesions appear as multiple, alopecic, non-healing scabs on the trunk, limbs, and head. 22 Histologically, MSCCIS does not invade the epidermal basement membrane, but in some cases, focal areas of invasive SCC are present. These areas are firm, crusted, crateriforme masses. 22
Nail bed SCCs are frequently incidental but may be accompanied by lameness, split toenails, and bleeding. 10 Eighty percent of dogs with digital SCC have radiographic evidence of bone lysis. 10,11 Multiple digital SCCs are occasionally seen in dogs. 10,11,20,21 In cats, nail bed carcinomas may represent metastasis from a primary lung carcinoma; most commonly, these cats are evaluated because of lameness, not respiratory signs. 23
Diagnosis and Staging
The diagnosis of SCC should be histologically confirmed, since inflammation may confound cytologic diagnosis. Three-view thoracic radiographs and a regional lymph node aspirate should be performed. Biopsy of superficial facial lesions may be accomplished by shaving the lesion with a scalpel blade. Since the resulting biopsy samples are thin and fragile, placement into a tissue cassette prior to submerging in formalin is indicated.
In dogs and cats with nail bed SCC, radiographs of the affected digit(s), three-view thoracic radiographs, and a regional lymph node aspirate are indicated to evaluate the patient for bony lysis of the digit and pulmonary metastasis or a primary bronchogenic carcinoma (cats). 10,11,23
Biological Behavior/Metastasis
Cutaneous SCC is locally invasive. Metastasis to regional lymph nodes is uncommon and pulmonary metastasis is rare. In a study of cats with planum SCC, 6 of 15 (40%) of necropsied cats had regional lymph node metastasis, and 1 (6%) had pulmonary metastasis. 13 Only 3% to 13% of dogs with nail bed SCC have radiographic evidence of pulmonary metastasis at the time of diagnosis, 10,11 and, in one study, 29% eventually developed metastasis. 10
Therapy and Prognosis
For dogs with solitary or multiple dermal SCC, surgical excision and behavioral modification to avoid sun exposure are recommended. When multiple lesions are present, topical imiquimod cream (Aldara, 3M Pharmaceuticals, St. Paul, MN) may slow the progression of dermal SCC and other skin lesions. 24 Although the extent of disease in most patients limits its use, electron beam radiation therapy could be considered for localized SCC. Anecdotally, laser removal 25 and oral COX-2 inhibitors have been used to control SCC lesions. Oral and topical retinoids are uncommonly used for multiple SCC in dogs because of cost, potential for serious side effects, and questionable efficacy. 26
For cats with SCC lesions on the face, treatment options are limited by the size and invasiveness of the tumor, so early treatment is best. Untreated SCC invades normal tissues, resulting in disfigurement and loss of function. 15 Pinnectomy and nosectomy can be effective in controlling the disease. 15 Tumor control is better for cats with tumors amenable to surgical excision when compared with external beam radiation therapy, and cryotherapy is associated with shorter control times when compared with either of these modalities. 15
Strontium-90 beta radiation ( 90 Sr) is effective for lesions < 2 mm in depth and incompletely excised small lesions ( Figure 23-2 ). 27,28 90 Sr is effective for superficial SCC, with nearly 90% of cats tumor free at 1 year and 80% tumor free at 2 years after therapy. 27 In a study of 90 cats with nasal planum SCC treated with external beam radiation therapy, 1- and 5-year progression-free rates were 60% and 10%, respectively. Cats with tumors < 2 cm had a better prognosis than those with tumors > 3 cm in diameter. 13 For more advanced local disease, external beam radiation therapy may provide long-term tumor control. 13,15 Intratumoral administration of carboplatin (Paraplatin, Bristol-Myers Squibb Co., Princeton, NJ), 29 photodynamic therapy, 30,31 and cryotherapy 15 have also been used for superficial lesions but are associated with shorter control times. SCC is poorly responsive to systemic chemotherapy. 15 In one study, none of the feline cutaneous SCC tested expressed COX-2, suggesting that COX-2 inhibition is unlikely to be useful in this species. 32
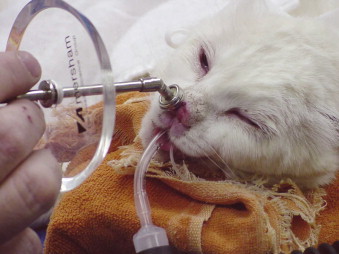 |
| FIGURE 23-2 Strontium-90 ( 90 Sr) beta irradiation is an effective treatment for superficial squamous cell carcinoma in cats. Here the 90 Sr probe is applied to a lesion on the nasal planum. |
Client education about SCC prevention is essential. Techniques to minimize sun exposure in dogs and cats include limiting access to the outdoors and windows during peak sun hours and the use of topical sunscreen or protective clothing.
Surgical excision can provide local control of MSCCIS lesions; however, cats frequently develop lesions at other sites. 22 In a recent report of 12 cats with MSCCIS treated with imiquimod, all cats showed improvement in lesions. 33 Observed toxicities included local erythema, gastrointestinal signs, elevated liver enzymes, and neutropenia. 90 Sr may also be useful for lesions < 2 mm in depth. 34 In cats with MSCCIS, local recurrence following surgical excision is rare; however, long-term monitoring is required because of the multifocal nature of the disease. 22 Long survival times are observed. 33
For dogs with nail bed SCC, wide amputation with disarticulation of the first phalanx or metacarpal/metatarsal bone is the treatment of choice. Radiation therapy and chemotherapy may be indicated for incompletely excised or metastatic tumors. 10 In one study of dogs with nail bed SCC, the 1- and 2-year survival rates were 95% and 74%, respectively, for subungual SCC, and 60% and 44% for SCC from other parts of the digit. 11 A more recent study showed a 1-year survival rate of 50% and a 2-year survival rate of 18%. 10 Only 15% had metastasis at the time of death or last follow-up, and dogs treated with surgery survived longer than those not treated.
For cats with nail bed tumors resulting from metastasis of primary bronchogenic carcinomas, surgical resection of the digit is rarely palliative because of the poor prognosis associated with this syndrome. 23 In one study, the mean survival time for these cats was 58 days from diagnosis, regardless of treatment. 23
Papilloma ( Table 23-3 )
| Papilloma | Basal Cell Tumor (Trichoblastoma; Epithelial Tumor Without Epidermal or Adnexal Differentiation) | Trichoepithelioma | Pilomatricoma | Trichilemmoma | Infundibular Keratinizing Acanthoma (Ika; Formerly Intracutaneous Cornifying Epithelioma) | |
|---|---|---|---|---|---|---|
| Tissue of origin | Benign proliferation of the epidermis | Benign tumor of hair germ; recently, immunohistochemistry has resulted in reclassification of basal cell tumors as the follicular tumors trichoblastoma and trichoepithelioma or as ductular sweat gland tumors 36,37 | Benign tumor of the hair follicle that differentiates into all three segments of the hair follicle; incomplete trichogenesis may be present 39 | Benign tumor of the hair bulb showing matrical differentiation ± dystrophic mineralization and bone formation 39,40 | Benign tumor of the outer root sheath of the hair follicle; a bulb type and an isthmus type have been described 35,38-40 | Benign tumor of squamous cells of the follicular isthmus with a central accumulation of keratin 40 |
| Incidence | Uncommon in dogs; rare in cats 7,8 | Most common pigmented tumor of cats; common in dogs 4,35-38 | Common in dogs; rare in cats 4,35,38-40 | Relatively common in dogs; rare in cats 4,35,38-40 | Uncommon, mainly in dogs 35,38-40 | Common in dogs 40,41 |
| Risk factors/etiology | Caused by species-specific papillomaviruses in young animals; may also be non-viral (squamous papilloma) 7,8 | No breed predilection in cats 4 ; predisposed canine breeds include Kerry blue terrier, soft coated Wheaton terrier, bichon frise, cock-a-poo, Shetland sheepdog, husky, cocker spaniel, poodle, Airedale terrier, English springer spaniel, collie, Yorkshire terrier, and mixed breed 36,37 ; most common in middle-aged animals | Predisposed breeds include Bassett hound, bull mastiff, English springer spaniel, golden retriever, Gordon Setter, Irish Setter, German shepherd, and standard poodle 35,39,40 ; most common in middle-aged to older dogs | Kerry blue terriers markedly predisposed; other predisposed breeds include soft-coated Wheaton terrier, standard poodle, Old English sheepdog, bichon frise, Airedale terrier, basset hound, miniature poodle, Lhasa apso, and miniature schnauzer 35,39,40 ; most common in middle-aged dogs | None known | Norwegian elkhounds are markedly predisposed and often develop multiple lesions; other predisposed breeds include Yorkshire terrier, Lhasa apso, bichon frise, German shepherd, standard poodle, keeshond, Samoyed, and Shetland sheepdog 39,40 ; most commonly seen in middle-aged dogs |
| Clinical features | Small, slow-growing, cauliflower-like, proliferations of squamous epithelium; single or multiple lesions possible; most commonly located on the head or paws 7-9 | Solitary, pigmented, well-circumscribed, cystic or solid masses 36-38 ( Figure 23-3 ) | Slow-growing solitary or multiple masses; most commonly found on the dorsum, neck, thorax, or tail 39,40 | Masses most common on the dorsum, neck, thorax, and tail 39,40 | Alopecic, well-circumscribed dermal or subcutaneous mass(es) 40 | Multiple variably sized masses; most commonly on the dorsum, tail, and neck; masses have a central pore filled with grayish-white inspissated keratinous material that is pasty 40 ; release of keratinous material results in inflammation and secondary infection is possible |
| Diagnosis and staging | Clinical presentation suggestive, but definitive diagnosis requires biopsy; intranuclear inclusion bodies are seen in viral-induced papillomas 7,8 | Cytology is suggestive, definitive diagnosis with biopsy | Cytology is suggestive, definitive diagnosis with biopsy | Cytology is suggestive, definitive diagnosis with biopsy | Cytology is suggestive, definitive diagnosis with biopsy | Cytology is suggestive, definitive diagnosis with biopsy |
| Therapy and prognosis | Generally, no treatment is required as most regress spontaneously within 1 year with associated infiltration of T cells 7,8 ; surgical excision indicated for inverted papilloma (endophytic variant) or squamous papillomas that are irritating or bleeding | Most cured with excision | Most cured with excision; some breeds (especially Bassett hounds) develop multi-centric lesions | Most cured with excision | Most cured with excision | Surgical resection for solitary lesions; dogs with multiple tumors are prone to developing more lesions and may benefit from long-term retinoid therapy 41 |
FOLLICULAR TUMORS
Multiple tumor types are associated with the hair follicle. 4,35 Most are solitary benign lesions that are diagnosed and cured with surgical excision and histologic examination. Benign follicular tumors are described in Table 23-3 . Their malignant counterparts (malignant trichoepithelioma and pilomatricoma) are rare and reported only in dogs. The exception is basal cell carcinoma (BCC), which is discussed subsequently.
Basal Cell Tumors
The term basal cell tumor describes epithelial tumors without epidermal or adnexal differentiation and has historically included a group of diverse epidermal, follicular, and adnexal tumors. In recent years, these tumors have been reclassified based on morphologic and immunohistologic features. 36,37 The majority are suspected to be trichoblastomas; however, to date, the term basal cell tumor remains in common use in veterinary histopathology. Basal cell tumors are common in cats and dogs. 4,35-38,42 ( Figure 23-3 ). See Table 23-3 for further information.
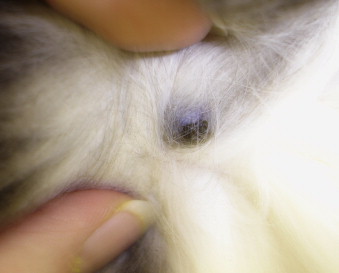 |
| FIGURE 23-3 Trichoblastomas (previously termed basal cell tumors ) are the most common pigmented tumor of the skin in cats. |
Basal Cell Carcinoma
Incidence/Etiology and Risk Factors
Basal cell carcinomas (BCCs) are uncommon, although their true incidence is unknown since many of these tumors were previously classified as basal cell tumors or have been confused with ductular adenomas or carcinomas. 43
Clinical Features
BCCs often appear as plaques or nodules, and may have a blue or black pigment caused by the presence of melanin. The overlying epidermis may be ulcerated. Multi-centric lesions are common in cats, usually occurring on the nose, face, and ears. In dogs, BCCs are usually truncal. 43
Diagnosis and Staging
The diagnosis may be obtained by cytology or biopsy. Regional lymph node aspiration and three-view thoracic radiographs are indicated, although the incidence of metastasis is low. 43
Therapy and Prognosis
The treatment of choice for BCC is wide surgical excision. Adjuvant radiation therapy may be indicated if excision is incomplete. The role of chemotherapy is unknown but may be considered if BCCs are metastatic. 44,45 Long-term survival is expected for completely excised lesions. Pulmonary and regional lymph node metastases have been reported in BCC. 45 The prognosis for metastatic BCC is unknown.
SEBACEOUS GLAND TUMORS
Sebaceous glands produce sebum, an oily white fluid. In addition to cutaneous sebaceous tumors, dogs and cats develop tumors of modified sebaceous glands including hepatoid gland tumors of the perianal region (see Section G ) and meibomian gland tumors ( Chapter 20, Section C ).
Incidence/Etiology and Risk Factors
Sebaceous adenoma, ductal adenoma, and epithelioma are benign tumors that are common in dogs and rare in cats. Sebaceous carcinoma is uncommon. 46,47 Sebaceous tumors are most common in middle-aged to older dogs and cats. Predisposed breeds include English cocker spaniel, cocker spaniel, Samoyed, Siberian husky, cock-a-poo, Alaskan malamute, West Highland white terrier, cairn terrier, dachshund, miniature poodle, toy poodle, shih tzu, and Persian cats. 46
Clinical Features
Sebaceous tumors may be solitary or multiple. Common sites are the head and dorsum. Sebaceous tumors present as elevated alopecic nodules that may contain keratin or may have a wart-like appearance. Sebaceous epitheliomas may be pigmented. 46-48
Diagnosis and Staging
Cytology may be suggestive of the diagnosis, and biopsy is needed for definitive diagnosis. Regional lymph node aspiration and three-view thoracic radiographs are indicated for sebaceous carcinomas.
Biological Behavior/Metastasis
Sebaceous carcinomas are locally invasive. Rarely, sebaceous carcinomas and epitheliomas metastasize to local lymph nodes or other sites. 46-48
Therapy and Prognosis
Wide surgical resection is curative for most sebaceous tumors. Rarely, sebaceous carcinomas and epitheliomas metastasize. 46-48
APOCRINE GLAND TUMORS
Apocrine glands are sweat glands typically associated with hair follicles. In addition to tumors of cutaneous apocrine glands, dogs and cats commonly develop tumors of modified apocrine glands including adenoma or adenocarcinoma of the apocrine gland of the anal sac (see Section G ), ceruminous gland adenoma or adenocarcinoma (see Chapter 20, Section D ), and mammary adenomas and adenocarcinomas (see Chapter 22, Section F ).
Incidence/Etiology and Risk Factors
Benign apocrine tumors, including apocrine cyst, cystadenoma, ductular adenoma, and secretory adenoma, are common in the dog and uncommon in the cat. Cutaneous apocrine gland carcinomas are uncommon. 49,50 Apocrine tumors are most common in middle-aged to older dogs. Predisposed breeds for adenomas include Lhasa apso, Old English sheepdog, collie, shih tzu, great Pyrenees, chow chow, malamute, and Irish setter; for carcinomas, they include the Old English Sheepdog, shih tzu, German shepherd, cocker spaniel, Coonhound, Norwegian Elkhound, and Siamese cats. 49,50
Clinical Features
Apocrine tumors usually present as solitary masses on the head (cats and dogs) and legs (dogs). 49,50 Multiple masses are also possible. 51 Tumors may be freely movable or invasive.
Diagnosis and Staging
Cytology may be suggestive of the diagnosis, and biopsy is needed for definitive diagnosis. Regional lymph node aspiration and three-view thoracic radiographs are indicated for apocrine carcinomas.
Biological Behavior/Metastasis
The growth rate of apocrine carcinomas is variable and metastasis is uncommon, usually to lymph nodes and lungs. Inflammatory carcinomas are associated with rapid growth and metastasis. 49
Therapy and Prognosis
Surgical resection is curative for most apocrine tumors. 51,52 In a study of 25 dogs with apocrine gland carcinomas treated with surgery, only 1 dog was euthanized for tumor-related causes and the median survival time (MST) was 30 months (17 dogs still alive). 51 Occasionally, lymphatic and distant metastasis may be seen. Metastasis is predicted by vascular invasion and tumor grade. 50,51 For incompletely excised carcinomas or those with vascular/lymphatic invasion or regional lymph node metastasis, adjunctive therapy with radiation or chemotherapy is indicated. Protocols for these tumors are not well described, but protocols effective for anal sac gland adenocarcinoma (see Section G ) or mammary adenocarcinomas ( Chapter 22, Section F ) would be logical choices.
MELANOCYTIC TUMORS
Incidence/Etiology and Risk Factors
Melanoma is common in dogs 4,53,54 and the second most common tumor of the digit in this species. 10,11,53,55,56 Melanoma is rare in cats. 4,5,53,56 The most common pigmented tumor in cats is basal cell tumor (trichoblastoma). Melanomas are more common in purebred dogs. 57 Genetic predisposition is suggested by increased prevalence in standard and miniature schnauzers, Doberman pinschers, Scottish terriers, Boston terriers, boxers, Airedale terriers, Irish and Gordon setters, cocker and Springer spaniels, and Golden retrievers. 58 More recently, for melanoma of the haired skin, Labrador retrievers, miniature schnauzers, and Rottweilers were overrepresented and for melanoma of the digit, Labrador retrievers, Golden retrievers, and Rottweilers were overrepresented. 59
Clinical Features
Although melanomas are typically pigmented dermal masses, some are amelanotic.
Diagnosis and Staging
Cytology may be suggestive of the diagnosis, but melanoma is diagnosed with biopsy and histology. Immunohistochemical stains (S100 and MelanA) for proteins expressed by melanomas may be required for diagnosing anaplastic tumors. 60 For dogs with negative prognostic indicators (i.e., digital or mucocutaneous junction location, malignant histologic appearance, high mitotic index, or vascular/lymphatic invasion), cytology of regional lymph nodes, and three-view thoracic radiographs are indicated. Abdominal ultrasound should also be considered. Because the behavior of cutaneous melanomas in cats is less predictable, these tests are recommended routinely.
Biological Behavior/Metastasis
Cutaneous melanoma is typically behaviorally benign in dogs. 61,62 Malignant melanoma metastasizes via lymphatics to lymph nodes, lungs, and other sites. The behavior of cutaneous melanomas in cats is less predictable. In cats, melanomas are often slow growing and some are cured with surgical excision, but others recur locally or spread to lymph nodes, lungs, and other sites, including viscera or bone. 63,64
Therapy and Prognosis
Most dogs with cutaneous melanomas are cured with complete surgical excision. 61,62 The histologic tumor grade is an important predictor of survival; in one study of 59 dogs treated with surgery for cutaneous melanoma, 10% of dogs with a mitotic index ≤ 2/10 HPF and 73% of dogs with a mitotic index ≥ 3/10 HPF died of melanoma within 2 years of surgery. 62 In a more recent study, only 12% of 227 cutaneous melanomas exhibited recurrence or metastasis, and it was difficult to predict which tumors would be more aggressive using histologic features. 61 Nuclear atypia was thought to be most reliable. For malignant melanomas of the canine digit, digital amputation is often required. This location is associated with more aggressive behavior, including local invasion and metastasis to lymph nodes, lungs, and other sites. For dogs with digital melanomas, reported 1- and 2-year survival rates are 42% to 44% and 11% to 13%, respectively. 10,11
Adjunctive therapies may be indicated for patients with malignant melanoma. Radiation therapy is effective for malignant oral melanomas in dogs ( Chapter 20, Section A ) and may be useful for control of incompletely excised malignant cutaneous melanomas and involved regional lymph nodes. Chemotherapy with carboplatin may be helpful for slowing local progression and metastasis. One study demonstrated a 28% response rate of measurable malignant melanomas to this drug. 65 In addition, because melanomas are highly immunogenic, there has been great interest in developing immunotherapy as a treatment for these tumors. 57,66,67 A xenogenic DNA canine melanoma vaccine is currently commercially available (Canine Melanoma Vaccine, Merial Limited, Duluth, GA). Plasmid DNA encoding human tyrosinase (a melanocytic protein) is injected into the muscles of the medial thigh. Canine myocytes express the DNA, and the resultant human tyrosinase induces an immune response. Human and canine tyrosinase are similar enough that the immune response can cross over to be directed at canine melanocytic cells. 66,68 This vaccine has been demonstrated to be safe and active in phase 1 and 2 clinical trials in dogs and survival times > 1 year have been described. It is recommended that this vaccine be used as adjunctive therapy once locoregional tumor control has been achieved. Currently, the canine melanoma vaccine is licensed for use by veterinary oncologists only.
The behavior of cutaneous melanomas in cats is much less predictable, and even histologic diagnosis as benign or malignant may not predict behavior. 63 In one study of cats with cutaneous melanoma treated with surgery with or without adjuvant therapies, survival times ranged from 0 to 1003 days, with 5 of 19 cats dying of melanoma. 63 Another study described 45 cats with cutaneous melanoma treated with surgery 64 ; 22 of 37 cats that died during the study had local recurrence. Sixteen of these 37 cats were necropsied and all had metastasis.
MESENCHYMAL TUMORS
Cutaneous mesenchymal tumors are usually STSs (see Section E ), a group of tumors with similar histologic and clinical features. Although they are mesenchymal tumors of soft tissue, cutaneous hemangiosarcomas (HSAs) are considered separate from STS because of their distinct etiology and behavior.
Hemangioma/Hemangiosarcoma
Incidence/Etiology and Risk Factors
HSAs and hemangiomas are tumors of vascular endothelium. They occur more frequently in dogs than any other species but are uncommon in the skin of dogs and less common in cats. 69,70 In dogs, cutaneous hemangioma is more common than HSA. Cutaneous HSA is more common in older animals. Predisposed breeds include the Italian greyhound, greyhound, whippet, Dalmatian, pit bull, boxer, and Basset hound. 69-71 Solar radiation induces dermal hemangiomas and HSA in dogs with short hair and lightly pigmented skin. 71 Subcutaneous HSA is not solar induced. The identification of pairs of affected sibling Italian greyhounds and whippets suggests a possible genetic predisposition. 70,71
Clinical Features
Dermal hemangiomas and HSAs appear as red or purple discolorations or raised masses, and lesions may be solitary or multiple. Dermal HSA frequently occurs in lightly haired and unpigmented areas, including the ventral abdomen, limbs, neck, dorsum, and head. 69-72 Subcutaneous lesions are firm or soft masses that often appear bruised and have no site predilection.
Diagnosis and Staging
Diagnosis of HSA is accomplished with biopsy and histology. For anaplastic tumors, immunohistochemical staining for Factor VIII–related antigen (von Willebrand factor), and CD31 can be used to confirm the diagnosis. 73,74 Solar-induced changes such as dermal elastosis and actinic keratosis may be observed in the skin surrounding dermal HSA. Because HSA of the skin can metastasize or may represent metastasis from visceral HSA, further staging tests are indicated. A CBC, serum chemistry profile, urinalysis, thoracic and abdominal radiographs, abdominal ultrasound, cytology of local lymph node, and echocardiogram are recommended, particularly for subcutaneous lesions, multiple lesions, or lesions not consistent with solar etiology. A staging scheme has been described for canine cutaneous HSA: stage I is a primary tumor confined to the dermis, stage II is a primary tumor involving the hypodermis, and stage III is a primary tumor with underlying muscular involvement. 69
Biological Behavior/Metastasis
Patients with solar-induced hemangioma and HSA can have multiple lesions and are at risk of developing future lesions. HSA is locally invasive and metastasis is uncommon with dermal HSA, but common with subcutaneous HSA. 69 Common sites are skin, lymph node, and lung.
Therapy and Prognosis
Most hemangiomas and dermal HSAs are cured with complete surgical excision, but additional lesions may develop and avoidance of sun exposure is important. Subcutaneous lesions require wide surgical excision. Completeness of excision is prognostic for survival of dogs and cats with cutaneous HSA treated with surgery alone or in combination with doxorubicin (Adriamycin, Ben Venue Laboratories, Inc., Bedford, OH). 70,75,76 In dogs with HSA, subcutaneous tumors have been associated with a poorer prognosis than dermal tumors; in one study, the median survival of dogs with stage I disease was 780 days compared with 172 and 307 days for stage II and III dogs. 69 However, with aggressive therapy and complete excision, good prognoses may be achieved for some dogs with subcutaneous HSA. 70 This is supported by a recent study that showed a median disease-free interval of > 4 years for dogs with subcutaneous HSA treated with surgery and doxorubicin with or without radiation therapy. 77 In the same study, four dogs with intramuscular HSA had a median DFI of 265 days.
Radiation therapy may improve local control of incompletely excised cutaneous HSA in dogs and cats, and patients with non-resectable tumors may benefit from palliative radiation therapy. Fourteen of 17 dogs with evidence of soft tissue involvement with HSA (including SC and IM tumors) had tumor reduction (including four complete responses) following a palliative course of radiation therapy with or without doxorubicin chemotherapy. 78 The MST was approximately 3 months.
For subcutaneous tumors or those with vascular/lymphatic invasion or metastasis, adjunctive chemotherapy may improve survival time. Treatment protocols are those used for splenic HSA ( Chapter 22, Section D ) and should include doxorubicin. In a study evaluating vincristine (Vincasar, SICOR Pharmaceuticals, Irvine, CA), doxorubicin, and cyclophosphamide (Cytoxan, Mead Johnson Oncology Products, Princeton, NJ) chemotherapy for dogs with HSA, an MST of 425 days was reported for 6 dogs with subcutaneous HSA, 79 and in a study evaluating doxorubicin and cyclophosphamide for dogs with HSA, survival times ranged from 183 to 704 days for four dogs with subcutaneous HSA. 80 In one study of 17 dogs with subcutaneous HSA treated with doxorubicin chemotherapy with or without radiation therapy, the median survival was 1189 days. 77 The role of continuous low-dose oral chemotherapy for subcutaneous HSA has not been defined, but a preliminary study of cyclophosphamide, etoposide (Etoposide, Gensia Sicor Pharmaceuticals, Irvine, CA), and piroxicam (Feldene, Pfizer Incorporated, New York, NY) administered in this fashion after splenectomy to dogs with splenic HSA demonstrated a similar MST (6 months) to dogs treated with doxorubicin. 81 There is much interest in immunotherapy and antiangiogenic therapy for HSA, 82,83 but these therapies are not readily available at this time.
In cats, subcutaneous HSA is more likely to recur or metastasize than cutaneous HSA, but complete excision can be associated with long survival. 72,76 In one study, 10 cats treated with surgery for subcutaneous HSA lived 13 to >112 weeks. 84 In another study of 18 cats with cutaneous HSA, the MST was 912 days (range, 4–1460; 5 cats still alive at 120–1186 days), and cats treated with surgery lived significantly longer than those not treated with surgery. 72 For incompletely excised HSA, radiation therapy should be considered. There is little information regarding adjuvant chemotherapy for HSA in cats, but doxorubicin (as a single agent or in combination with vincristine and cyclophosphamide), carboplatin, and a combination of mitoxantrone (Novantrone, Immunex Corp, Seattle,WA) and cyclophosphamide have been used. 72,76,85
ROUND/DISCRETE CELL TUMORS
Round/discrete cell tumors exfoliate as individual round cells and have distinguishing cytologic features that frequently allow diagnosis. Tumors included in this group are MCT (see Section D ), lymphoma (see Chapter 25, Section A ), plasmacytoma (PCT), histiocytoma, and canine transmissible venereal tumor (CTVT; see F and H ). Any of these tumors can occur in the skin.
Plasmacytoma
Incidence/Etiology and Risk Factors
Plasma cell tumors are uncommon in the skin of dogs and are very rare in cats. They are more common in older animals, and cocker spaniels may be overrepresented. 86,87
Clinical Features
Plasmacytomas appear as raised, hairless, pink dermal nodules and are typically found on the face, ears, or feet ( Figure 23-4 ). 86,87 In dogs, they are usually solitary, but multiple PCTs are possible.
 |
| FIGURE 23-4 Cutaneous plasma cell tumors frequently appear as pink, alopecic lesions on the face, ears, or feet. This dog was treated with chemotherapy for multiple cutaneous plasmacytomas and lived > 3 years, before dying of unrelated causes. |
Diagnosis and Staging
Cytology is often diagnostic and histology provides a definitive diagnosis. For more anaplastic tumors, immunohistochemical staining may be required to definitively differentiate PCT from lymphoma or MCT. For most dogs, a CBC, serum biochemical profile, urinalysis, and regional lymph node aspirate provide complete staging information. For dogs with multiple tumors, lymph node metastasis, or hyperglobulinemia, serum and urine protein electrophoresis or immunoelectrophoresis, thoracic and abdominal radiographs (including axial skeleton), abdominal ultrasound, and bone marrow aspirate are indicated. Because of the systemic nature of PCT in cats, all of the staging tests listed are recommended.
Biological Behavior/Metastasis
Most cutaneous PCTs in dogs are primary tumors. 86,87 Rarely, canine cutaneous PCT may be associated with multiple myeloma and/or paraneoplastic syndromes (including hyperglobulinemia and hypercalcemia). 86,87 In contrast, in cats PCT may be more commonly associated with metastasis or multiple myeloma. 88,89
Therapy and Prognosis
Most cutaneous PCTs in dogs are benign and are cured with surgical excision. 86,87 In one study of 57 dogs with cutaneous PCT, 46 had no recurrence at 1 to 62 months after surgery, and 4 were euthanized for systemic disease related to the tumor. 87 Histologic grading of canine PCT does not predict behavior. 90 Rarely, dogs develop multiple cutaneous PCTs or lymph node metastasis. These dogs may enjoy long survival times with chemotherapy. A chemotherapy protocol for multiple myeloma (combination of melphalan [Alkeran, Cardinal Health for GlaxoSmithKline, Albuquerque, NM] and prednisone; see Chapter 25, Section C ) is indicated for cats and for dogs with metastatic disease or multiple PCTs. Radiation therapy can provide long-term control for tumors in locations not amenable to surgery. There is little information describing the prognosis for PCT in cats, but association with systemic disease would suggest a poorer prognosis. 88
Histiocytoma
Incidence/Etiology and Risk Factors
Histiocytic proliferative disorders (HPDs) of dogs include cutaneous histiocytoma, reactive histiocytosis (cutaneous and systemic histiocytosis [believed to be an immunoregulatory disorder]), and malignant histiocytosis (localized and disseminated histiocytic sarcoma [HS] [ Section F ]. These diseases are distinguished by histologic appearance, immunophenotype, and varying clinical presentations/courses. 91-94 Histiocytomas are benign tumors of Langerhans’ cells (antigen-presenting cells of the epidermis). They are most common in young, purebred dogs but can be seen in any dog. 95 Breeds at risk include Scottish terriers, boxers, Doberman pinschers, Labrador retrievers, cocker spaniels, Rottweilers, and miniature schnauzers. 95 They are extremely rare in cats.
Clinical Features
Histiocytomas are hairless, raised, and white, pink, or red dermal nodules that can become inflamed and ulcerated, especially when regressing ( Figure 23-5 ). Most commonly they occur on the head or limbs.
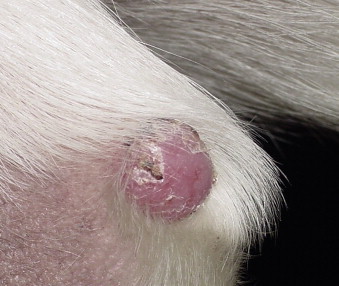 |
| FIGURE 23-5 Histiocytomas frequently appear as pink alopecic lesions on the limbs of young dogs and spontaneously regress within a few months. |
Diagnosis and Staging
Cytology is usually diagnostic for histiocytoma. Because of the possibility of misdiagnosis of cutaneous lymphoma or another round cell tumor, in cases with an atypical presentation (old dog, multiple lesions, not regressing), biopsy and histologic confirmation of diagnosis are indicated. Rarely, immunophenotyping 91-93 may be necessary to rule out other diagnoses and confirm that a mass is a histiocytoma.
Biological Behavior/Metastasis
Histiocytomas are usually solitary and do not metastasize; however, multiple regressing cutaneous histiocytomas have been reported. Langerhans cell histiocytosis (LCH) is a syndrome of multiple cutaneous histiocytomas that may be slow to regress, wax and wane for a period, or progress to organ involvement. 96,97 LCH is seen in children and has been reported in young dogs.
Therapy and Prognosis
Most canine histiocytomas regress spontaneously within 2 to 4 months, and recurrence or development of additional lesions is rare. If a histiocytoma does not resolve, is irritating to the patient, or has an atypical presentation, surgical resection and histology are indicated.
k Alkeran®, Cardinal Health for GlaxoSmithKline, Albuquerque, NM.
Selected References ∗
J.P. Bergman, M.A. Camps-Palau, J.A. McKnight, et al. , Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center , Vaccine 24 ( 2006 ) 4582 ;
This article presents details of the development, efficacy, and safety of the first tumor vaccine licensed for treatment of cancer in veterinary patients, the canine melanoma vaccine. .
E.J. Bulakowski, J.C. Philibert, S. Siegel, et al. , Evaluation of outcome associated with subcutaneous and intramuscular hemangiosarcoma treated with adjuvant doxorubicin in dogs: 21 cases (2001-06) , J Am Vet Med Assoc 233 ( 1 ) ( 2008 ) 122 ;
This study describes a good prognosis for dogs with subcutaneous hemangiosarcoma if treated aggressively. .
In: (Editors: T.L. Gross, P.J. Ihrke, E.J. Walder, et al. ) Skin diseases of the dog and cat: clinical and histopathologic diagnosis ed 2 ( 2005 ) Blackwell Science , Oxford, England ;
This is a useful text providing detailed information regarding cutaneous neoplasia and is particularly useful for benign and rare skin tumors, topics for which little literature is available. .
G.M. Hammond, I.K. Gordon, A.P. Theon, et al. , Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990-2006) , J Am Vet Med Assoc 231 ( 5 ) ( 2007 ) 736 ;
This study demonstrates that strontium-90 is a safe and effective treatment for superficial squamous cell lesions in cats. .
C.M. Johannes, C.J. Henry, S.E. Turnquist, Hemangiosarcoma in cats: 53 cases (1992-2002) , J Am Vet Med Assoc 231 ( 2007 ) 1851 ;
This is the largest study to date describing the behavior of hemangiosarcoma in cats. .
W.L. Spangler, P.H. Kass, The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia, , Vet Pathol 43 ( 2006 ) 136 ;




This study examines prognostic indicators for predicting the behavior of melanocytic tumors. .
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


