Chapter 7. The Cytology of Neoplasia
Marlyn S. Whitney and Linda M. Berent
Cytologic examination of masses, effusions, and lymph nodes can aid in the diagnosis and staging of neoplastic processes. It is a rapid and relatively inexpensive procedure. However, it does have its limitations. Although cytology has excellent sensitivity and specificity for the round cell tumors, it is often equivocal in lesions complicated by inflammation or necrosis. Even when cytologic evaluation indicates that malignancy is present, it is often not possible to determine the specific type of carcinoma or sarcoma that is present. 1,2 Immunocytochemistry is not widely available and tumor grading cannot be accomplished using cytology alone. For these reasons, cytology is a useful tool in veterinary oncology but cannot completely replace histopathology. This chapter is intended to serve as an overview of common cytologic findings in neoplastic lesions and is not intended to be a stand-alone resource. A practitioner must be versed in the normal cytology for each tissue before he or she can competently diagnose neoplastic lesions. The reader is directed to any one of the excellent atlases available for a complete review of normal, inflammatory, and neoplastic cytology. 3-5
GENERAL APPROACH TO THE INTERPRETATION OF CYTOLOGIC SPECIMENS
Adequate samples are essential for optimal cytologic evaluation (see Chapter 6 ). The first step in slide evaluation is to locate an area with adequate cellularity and good quality of cells. The next step is to determine if the specimen is comprised primarily of inflammatory cells, non-inflammatory cells, or a mixture of both. Inflammatory cell populations are further classified according to the types of cells present, since this helps to narrow the list of etiologic possibilities. Non-inflammatory cell populations are categorized as one of the following: (1) normal cells for the given anatomic site, (2) hyperplasia or benign neoplasia (these often cannot be cytologically differentiated from one another), or (3) malignant neoplasia. Mixed cell responses are quite common and present as inflammatory cells mixed with normal, hyperplastic, or neoplastic cells. When cells are suspected of being neoplastic, the next step is to determine the general cellular category: epithelial cells, mesenchymal cells, or one of the discrete round cell neoplasms. Epithelial cells are round to polygonal and have a tendency to arrange in cohesive clusters. Mesenchymal cells often have one or more cytoplasmic tails. Those with one cytoplasmic tail may be described as flame shaped, those with two as spindle shaped, and those with more than two as stellate shaped. In some mesenchymal neoplasms, such as the lipoma, the cells may be round to oval. Mesenchymal cells tend to appear as single forms, but aggregates of cells may be present. The discrete round cell neoplasms, which are technically of mesenchymal origin, are usually placed into a separate category because they are made up of round cells that do not form cohesive clusters. Melanomas are of neuroectodermal origin and do not fit well into the above morphologic classification system, since their cells may exhibit round, epithelial and mesenchymal characteristics. The key to diagnosing a melanoma is to find melanin granules in the cytoplasm of the tumor cells.
CYTOLOGIC CRITERIA OF MALIGNANCY
Cytologic criteria of malignancy are primarily used to determine if an epithelial or mesenchymal population is abnormal enough to be considered malignant ( Box 7-1 ). These criteria often relate to the variability in the cell population and are less useful for monomorphic round cell neoplasms. General criteria of malignancy include anisocytosis (variable cell size in a population that would normally consist of cells of similar size), higher cellularity than expected, and presence of cells in an abnormal location. Nuclear criteria of malignancy include variable nuclear size (anisokaryosis), increased nuclear to cytoplasmic volume ratios (N:C ratios), variable N:C ratios in a cell population not normally expected to exhibit this feature, multiple nucleoli, variable size of nucleoli, presence of abnormally shaped nucleoli, coarse and irregular chromatin clumping, thickening of the nuclear margin, large numbers of mitotic figures, mitotic figures of abnormal configuration, presence of multiple nuclei, and nuclear molding. Nuclear molding is characterized by the wrapping of a nucleus from one cell around an adjacent cell, or the wrapping of one nucleus around another in a multi-nucleated cell. Cytoplasmic criteria of malignancy are the least useful and include increased cytoplasmic basophilia and vacuolation. As a rule, at least four general or nuclear criteria of malignancy should be observed before assigning the term malignant , although there are exceptions to this rule. One can be most confident in assessing malignancy when many criteria are present, or when one or more very strong criteria are present. The presence of cells in an abnormal location, such as sheets of epithelial cells within a lymph node aspirate, is such a strong criterion of malignancy that it can be used to make the assessment of malignancy even when few or no other criteria are present.
BOX 7-1
CYTOLOGIC CRITERIA FOR MALIGNANCY
Strong Criteria for Malignancy
• Cells in an abnormal location
• Nuclear molding
• Abnormal mitotic figures
• Odd numbers of nuclei
• Anisokaryosis within a multinucleate cell
• Variability in nucleolar size
• Abnormally large and/or irregularly shaped nucleoli
Weak Criteria for Malignancy (Often Also Seen in Hyperplastic Populations)
• Anisocytosis
• Anisokaryosis
• Cytoplasmic basophilia
• Normal mitotic figures
• Binucleation
Some of the criteria of malignancy just mentioned can also be found in hyperplastic cell populations. These include high cellularity, anisocytosis, anisokaryosis, increased N:C ratios, presence of multiple nuclei and/or nucleoli, increased mitotic figures, and increased cytoplasmic basophilia. Some types of epithelial cells, including squamous and transitional epithelial cells, normally exhibit variability in N:C ratios, because nuclei become smaller as the cells mature. When multiple nuclei are present in hyperplastic cells, they should be present in even numbers and should all be of equal size. While multiple nucleoli are often present in hyperplastic cells, they should remain small and round.
CYTOLOGIC APPEARANCE OF MESENCHYMAL TUMORS ( FIGURE 7-1 )
As a rule, mesenchymal neoplasms do not exfoliate well, often resulting in suboptimal cytology preparations. 6,7 Lipomas are the most common mesenchymal tumor in older dogs and often yield poorly cellular, oily material that will not dry when placed on slides. Rarely, mature adipocytes can be found and are characterized by large round cells with a large amount of clear cytoplasm and a small condensed oval nucleus at one pole. This cytologic appearance is identical to that of subcutaneous fat and should, therefore, always be correlated with the clinical presentation of the lesion. Liposarcomas are rare and have small clear cytoplasmic vacuoles in cells with features similar to the soft tissue sarcomas described in subsequent sections.
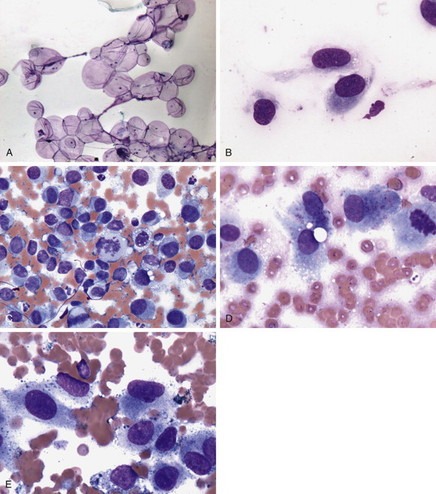 |
| FIGURE 7-1 A , Lipoma. Cytology preparations from lipomas consist of variable numbers of large round cells with small eccentric nuclei and abundant clear cytoplasm (fat cells, or adipocytes). The cells may be seen in aggregates or as single forms. Because fat cells do not adhere well to glass, many of the cells may be lost from the slide during the staining process and stained preparations thus may be quite hypocellular (Wright-Giemsa stain, 100×). B , Sarcoma. Sarcomas tend to be made up of cells with cytoplasmic tails that appear as individual forms rather than in cohesive sheets (Wright-Giemsa stain, 1000×). C , Malignant histiocytosis, splenic aspirate, dog. Malignant histiocytic neoplasms may have highly variable appearance. Discrete round cells that exhibit morphologic criteria of malignancy and that lack cytoplasmic granules often predominate, and such cells may exhibit phagocytosis of erythrocytes or other cells (not seen here). Spindle-shaped cells may also be present in some forms of malignant histocytic tumors. The lymphocytes seen here are most likely a component of the normal splenic tissue that remains (Wright-Giemsa stain, 600×). D , Osteosarcoma. The cells in osteosarcoma may vary from round to flame, spindle, or stellate shaped. Nuclei are round to oval, and the variably abundant basophilic cytoplasm may contain fine pink to red granular material, as seen here. The cells usually exhibit cytologic criteria of malignancy. Here, note anisocytosis, anisokaryosis, variability in N:C ratios, and multiple nucleoli (Wright-Giemsa stain, 1000×). E , Malignant melanoma. The cells in a melanoma may be epithelioid, spindle-shaped, appear as discrete round cells, or there may be a mixture of cell types, as seen here. The key to diagnosis is the presence of intracytoplasmic melanin granules. In benign melanomas and well-differentiated malignant ones, heavy granulation may obscure cellular detail. In malignant melanomas, granulation may range from heavy to scant to nearly non-existent (amelanotic melanoma). This melanoma has scant to moderate granulation. Note the anisocytosis, anisokaryosis, variability in N:C ratios, coarsely stippled chromatin patterns, and multiple nucleoli. A binucleate cell is present near the right margin of the field (Wright-Giemsa stain, 1000×). |
Soft Tissue Sarcomas
Soft tissue sarcomas are characterized by flame- to spindle- or stellate-shaped cells with a tendency to appear as individualized cells, rather than in cohesive groups. Multiple criteria for malignancy are usually present in sarcomas, differentiating them from the benign connective tissue tumors such as a fibroma. Cytologic examination may narrow the diagnosis to sarcoma, but it is often insufficient to further classify these lesions into subtypes of tumor (e.g., fibrosarcoma vs. hemangiopericytoma). Thus, a biopsy is necessary for definitive diagnosis. Care must be taken when assessing mesenchymal cells via cytology because reactive connective tissue cells may be misinterpreted as neoplastic. 8
Histiocytic Neoplasia
The nomenclature for histiocytic neoplasms is evolving based on new information generated almost daily. As of this writing, the most current information can be found at http://www.histiocytosis.ucdavis.edu . In addition to benign histiocytomas of young dogs, there are several highly aggressive malignancies of histiocytic origin. Cytologic features include bizarre spindle or polygonal cells with marked criteria of malignancy. Erythrophagocytosis is a unique feature of these malignant cells but is not consistently found in all cases.
Osteosarcoma
The cells of osteosarcoma (OSA) may vary from round to flame to spindle shaped. Nuclei are generally round to oval, and the cells usually exhibit numerous cytologic criteria of malignancy. The cytoplasm is usually moderately abundant and basophilic and may contain variable numbers of small pink to red granules. The cells sometimes appear to be embedded in a bright pink-staining matrix material, which may represent osteoid. Tumor cells may contain two or more nuclei, and nuclei of disparate size may be present in a single cell. Low numbers of multinucleate cells in which all nuclei are of equal size may be present, typical of normal osteoclasts that are admixed with the tumor cells. OSAs may be differentiated from other mesenchymal tumors by staining the air-dried slide for alkaline phosphatase activity. 9 This stain will react with the cytoplasm of the osteoblast, producing a dark brown to black reaction. The stain must be applied to unstained air-dried preparations of sufficient cellularity. Reactive bone formation, which might occur, for example, following a fracture or other damage to the bone or periosteum, can falsely mimic OSA in cytology preparations and will also stain positively for alkaline phosphatase activity. As such, it is critical that the cells are scrutinized for criteria of malignancy prior to the decision to use this stain. Radiographic findings and clinical history will often help prevent mistaking reactive bone for OSA.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


