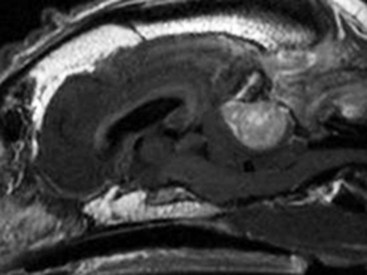
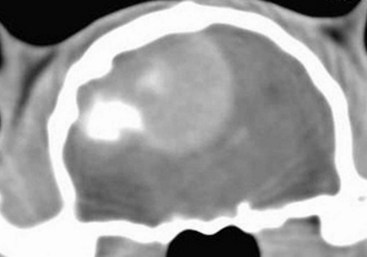
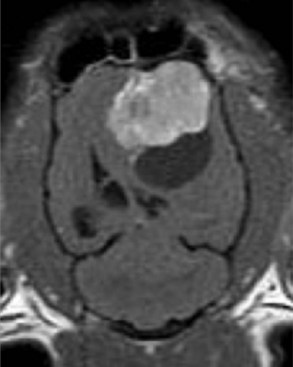
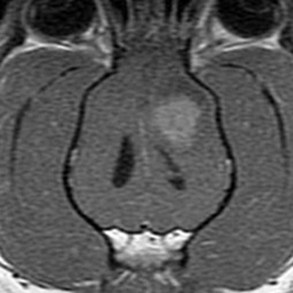
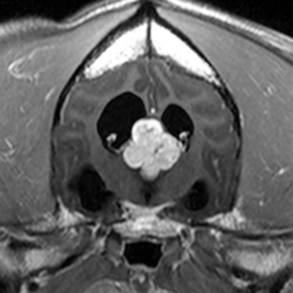
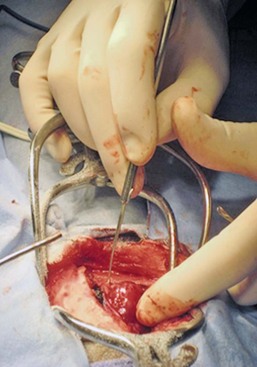
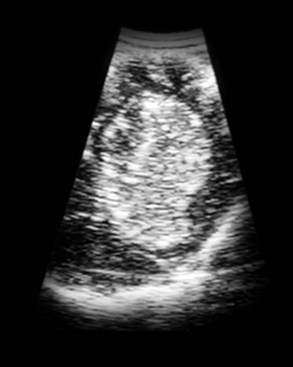
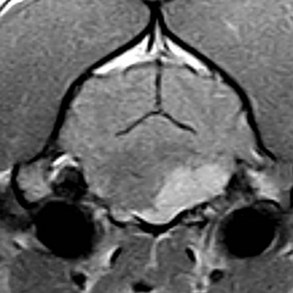
30 Brain tumors are frequently encountered in dogs and cats, with reported incidence rates of 14.5 dogs and 3.5 cats per 100,000 pets at risk, respectively.1,2 In addition to being more common in dogs than in cats, there is a wider variety of canine brain tumors than reported in cats; in addition, there is a wider range of histologic subtypes of canine meningioma reported compared with feline meningiomas.3,4 Most brain tumors encountered in clinical practice are primary tumors originating from brain parenchyma (e.g., glial cells and neurons, cells that line the interior and exterior brain surfaces [such as meningeal and ependymal cells]) or cells from vascular structures (e.g., choroid plexus). Meningioma is the most frequently reported primary brain tumor in dogs and cats, although gliomas are also frequently encountered in dogs.2,5–7 More than 50% of reported feline brain tumors are meningiomas.2,5,6 Gliomas are occasionally reported in cats.2,5 Other primary brain tumors less commonly reported in dogs include choroid plexus tumors; primary central nervous system (CNS) lymphosarcomas; primitive neuroectodermal tumors (PNETs), which typically includes neuroblastoma; primary CNS histiocytic sarcomas (also termed malignant histiocytosis); ependymomas; and vascular hamartomas. Other primary brain tumors occasionally encountered in cats include (in addition to gliomas): ependymomas, olfactory neuroblastomas, and choroid plexus tumors.2,5,7 In both dogs and cats, there are case reports of medulloblastomas, usually involving the cerebellum. Microglial tumors are considered rare in dogs and cats.1–3 Primary brain tumors in dogs and cats are typically solitary. Approximately 17% of cats with intracranial meningioma have more than one tumor.8,9 Multiple masses of the same primary brain tumor type in dogs are typically associated with choroid plexus tumors; in this scenario, tumor cells are disseminated or “dropped” via cerebrospinal fluid (CSF) flow in the ventricular system (“drop” metastases).5 The authors have encountered a number of Boxers with multiple intracranial gliomas. In one study, 10% of cats were found to have two different types of intracranial neoplasia concurrently.2 The occurrence of more than one histologic tumor type in dogs is considered rare. Secondary brain tumors include metastatic neoplasia, as well as tumors that affect the brain by local extension. Examples of metastatic neoplasia include mammary, pulmonary, and prostatic carcinoma; hemangiosarcoma; malignant melanoma; and lymphosarcoma. Tumors that may extend into the brain from the periphery include nasal and frontal sinus carcinoma (adenocarcinoma, squamous cell carcinoma), calvarial tumors (e.g., osteosarcoma, chondrosarcoma, multilobular osteochondrosarcoma), pituitary tumors (e.g., pituitary macroadenomas in hyperadrenocorticism), and nerve sheath tumors (e.g., cranial nerve [CN] V tumors). In one large retrospective study of secondary brain tumors in dogs,10 the most common secondary brain tumor was hemangiosarcoma (29%), followed by pituitary tumors (25%), lymphosarcoma (12%), metastatic carcinoma (11%), and invasive nasal tumors (6%). In one report,2 secondary (multicentric) lymphoma and pituitary tumors were the second and third most common intracranial neoplasms found in cats. Lymphoma/lymphosarcoma is considered the most common secondary brain tumor type encountered in cats. Brain tumors in dogs and cats can occur at any age, in either sex, and in any breed. However, brain tumors occur most commonly in older patients, with a median age of 9 years for dogs and older than 10 years for cats.2,5,6 Compared with other tumor types, dogs and cats with meningioma tend to be diagnosed at older ages.2,5–7 Male cats are more likely to develop intracranial meningioma than female cats.5,6 Golden retrievers and Boxers are considered to be predisposed to developing primary brain tumors.5–7 In general, dolichocephalic dog breeds are prone to develop meningiomas, whereas brachycephalic dog breeds are more likely to be afflicted with gliomas.5–7 There is no known breed predilection for cats to develop primary brain tumors.2,5,6 Cerebral tumors are more commonly encountered than tumors in the brainstem or cerebellum. Gliomas have a tendency to occur in the diencephalon and cerebellum, however.5,6 As mentioned previously, brain tumors are broadly classified as primary (neoplastic cells originating from tissues intrinsic to the brain, its vasculature, and meninges/ependyma) or secondary (neoplastic cells originating from tissues extrinsic to those comprising primary brain tumors).5 Brain tumors exert their pathologic effects both by directly encroaching on and/or invading brain tissue and by secondary effects, such as peritumoral edema, inflammation, obstructive hydrocephalus, and hemorrhage. The terms benign and malignant have different connotations when used in the context of intracranial neoplasia compared with tumors in other locations. Even slow-growing, readily removable brain tumors are not benign when considering the potential effect on the patient if therapy is not aggressively pursued in a timely fashion. Cytologic features of tumors are used to assess potential benignity or malignancy and include invasiveness, number of mitotic figures, and nuclear pleomorphism. Meningiomas are often regarded as benign. Considering the aggressive cytologic nature of many canine meningiomas (in comparison with human and feline meningiomas), this is probably an overstatement for this tumor type—especially in dogs.6,11 There are multiple histologic subtypes of meningiomas frequently encountered in dogs, including meningothelial, transitional, fibroblastic, psammomatous, angiomatous, microcystic, papillary, granular cell, myxoid, and anaplastic.3,4,6,11 In addition to these histologic subtypes, a grading system used in humans to predict the biologic behavior of intracranial meningiomas has recently been adapted to dogs; this grading system includes grade I (benign), grade II (atypical), and grade III (malignant).11 The repertoire of feline meningioma histologic subtypes is comparatively limited; the majority of feline meningiomas are meningothelial or psammomatous.2–4,6 Gliomas in dogs and cats include astrocytomas, oligodendrogliomas, and glioblastoma multiforme, in addition to undifferentiated gliomas. Gliomas as a group are considered to be malignant because these tumors tend to invade tissue and are typically resistant to all forms of definitive therapy.2,4,5,7,12 Clinicopathologic features of choroid plexus tumors in dogs have recently been reviewed.13 Choroid plexus tumors comprise choroid plexus papillomas (CPP) and choroid plexus carcinomas (CPC). Choroid plexus tumors in general tended to occur in the fourth ventricle in one report, and only CPCs occurred in the lateral ventricles.14 The two tumor subtypes are differentiated based on the presence or absence of local or distant metastases, as well as on histopathologic features. In one large study,7 it was found that half of canine primary brain tumors occupy more than one anatomic region of the brain; this could lead to the false conclusion based on neurologic examination that a patient with a solitary brain mass has multifocal disease. Also in that study, it was found that 23% of dogs with primary brain tumors had concurrent, unrelated neoplasia (e.g., pulmonary carcinoma, hemangiosarcoma), most of which involved the thoracic or abdominal cavity; this finding underscores the importance of screening for concurrent unrelated neoplasia (via thoracic radiography and abdominal ultrasonography) prior to pursuing advanced diagnostics and definitive therapy for the brain tumor. The diagnosis of brain tumor should be highly suspected in an elderly dog or cat with slowly progressive signs of brain dysfunction. A brain tumor should also be suspected in animals that experience a recent onset of seizure activity after 5 years of age, especially in certain breeds (e.g., golden retrievers, Boxers). Depending on the location and size of the tumor, such patients may appear neurologically normal interictally. Historic and presenting clinical signs for dogs and cats with brain tumors are variable and reflect both the location and the secondary effects (e.g., edema, hemorrhage) of the tumor. Seizures represent the most common presenting clinical sign of neurologic dysfunction in dogs with brain tumors, occurring in approximately half of the patients.5,6,15 In one study of feline brain tumors, the overall incidence of seizure activity was 23%, occurring more commonly with glioma (26.7%) and lymphoma (26.3%), in comparison with meningioma (15%).16 Cats with brain tumors are commonly presented to the veterinarian with a complaint of behavior change. In cats with primary brain tumors, nonspecific presenting clinical signs (i.e., signs not obviously referable to neurologic dysfunction) are fairly common, occurring in over 20% of cats in one large study.2 These clinical signs included lethargy, inappetence, and anorexia. Also in that study, it was found that approximately 19% of feline brain tumors were considered an incidental finding. Cerebral tumors are more common than tumors of the brainstem or cerebellum. Cerebral and diencephalic tumors tend to cause clinical signs of dysfunction such as seizure activity, behavior changes, circling, head-pressing, visual deficits, and hemiinattention (hemineglect) syndrome (ignoring environmental cues from the side opposite the tumor). Proprioceptive placing deficits and neck pain are often appreciable on neurologic examination. Tumors of the brainstem from midbrain through medulla often cause alterations of consciousness, dysfunction of cranial nerves (other than CN I and CN II), and obvious gait/proprioceptive abnormalities. Cerebellar tumors may result in clinical signs of dysfunction such as ataxia, dysmetria, intention tremors, vestibular abnormalities, and menace reaction deficits with normal vision.5,6 In most cases, clinical signs of neurologic dysfunction occur slowly and insidiously over time, especially with meningiomas. Owners of pets with meningiomas will often retrospectively realize that their pet had a behavior change for months to over a year prior to diagnosis. The subtle behavior changes are often attributed to “old age.” However, brain tumor patients can have subacute to acute development of neurologic dysfunction. These patients may experience sudden exhaustion of brain compensatory mechanisms or may suffer hemorrhage or acute obstructive hydrocephalus due to the tumor.5,6 Radiographs of the skull typically do not provide useful clinical information in cases of brain tumor. Computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used in the diagnosis of brain tumors, with MRI being the preferred imaging modality. Although specific types of brain tumors can vary in their appearance with these imaging modalities, there are some characteristic features that help distinguish meningiomas from gliomas. Meningiomas tend to have a broad-based, extraaxial attachment (they arise from the periphery of the brain and move inward, or axially), exhibit distinct tumor margins, and uniformly contrast enhance (Figure 30-1). Meningiomas tend to displace, rather than invade, parenchymal tissue; as discussed previously, however, many canine meningiomas do display some degree of invasiveness. Some meningiomas will calcify, which can be appreciated on a noncontrast CT image (Figure 30-2). The “dural tail” sign is an MRI feature typically associated with meningiomas, in which a contrast-enhancing meningeal-associated “tail” is seen extending from the main tumor mass (Figure 30-3). Meningiomas also may occasionally have a cystic component (cystic meningioma) extending from the main tumor mass (Figure 30-4). Gliomas tend to arise from an intraaxial location (from within the substance of the brain, moving outward), often lack distinct tumor margins (they tend to infiltrate, rather than displace normal tissue), and typically contrast enhance poorly and nonuniformly (Figure 30-5). Choroid plexus tumors and ependymomas tend to be intraventricular in location and often uniformly contrast enhance (Figure 30-6). The phenomenon of “ring enhancement,” in which a circular ring of contrast enhancement surrounds nonenhancing tissue, is nonspecific and has been associated with several neoplastic and nonneoplastic brain diseases. However, ring enhancement is often associated with gliomas (see Chapter 6). Meningeal contrast enhancement evident on MR images of the brain has been described but is not specific for brain tumors. These typical imaging features are guidelines only. Meningiomas can arise from the falx cerebri or the choroid plexus and appear intraaxial. Gliomas can be peripherally located and contrast enhancing. In a recent study, the accuracy of predicting primary brain tumor type based on MR images of 40 dogs was 70%.17 Stereotactic biopsy of brain tumors using MRI or CT images is now available at several veterinary referral centers.5 With this new technology, a definitive diagnosis can be obtained at the time of imaging without the need for major intracranial surgery. Figure 30-3 Postcontrast MRI of canine meningioma at the cerebellomedullary angle demonstrating a meningeal tail. The utility of CSF evaluation for the suspected brain tumor patient is controversial. CSF is often abnormal in patients with brain tumors, but the white blood cell (WBC) counts and protein levels are variable and nonspecific for neoplasia. In fact, dogs and cats with meningiomas tend to have CSF with predominantly polymorphonuclear (neutrophilic) WBC counts. The authors often do not pursue CSF analysis if the CT or MR image strongly suggests a brain neoplasm. Although the risk of CSF procurement in the face of elevated intracranial pressure (ICP) in a brain tumor patient is often not great, the potential benefit of a nonspecific CSF result may not outweigh even a small danger of harming the patient with the procedure. Although CSF analysis tends to yield fairly nonspecific information in brain tumor cases, it may be helpful in distinguishing whether a choroid plexus tumor is a CPP or CPC. In one study,13 the more malignant CPC was significantly more likely to be associated with a CSF protein concentration greater than 100 mg/dL than the less malignant CPP tumor subtype. Regardless of whether CSF analysis is performed, imaging should always precede CSF analysis when a focal neoplasm is highly suspected. Anesthetizing a patient who is most likely to have a brain tumor solely for the purpose of obtaining CSF is generally contraindicated because the resultant information is unlikely to assist in either planning treatment or estimating prognosis. In addition to removing neoplastic tissue, surgical debulking/removal allows for a histologic diagnosis, as well as potentially providing an immediate decompressive effect (decreasing ICP). Feline meningiomas are typically located over the cerebral convexities and tend to “peel away” from normal brain tissue at surgery. In most cases, feline meningiomas can be relatively easily removed en masse. The authors have had considerable experience dealing with recurrent or repeat feline intracranial meningiomas; overall, these tumors are also typically readily removable. Surgical removal is the primary mode of definitive therapy for feline intracranial meningiomas.2,5,6,9 Canine meningiomas are also often located over the cerebral cortical surface and are thus surgically accessible (Figure 30-7). However, meningiomas in the cerebellar and brainstem regions are frequently encountered in dogs. Cerebellar meningiomas are often surgically accessible; meningiomas in the brainstem may not be accessible. Meningiomas in dogs are much less predictable than those in cats in terms of ease of surgical removal. Unlike cats, there are multiple histologic subtypes of canine meningiomas, and nearly one-third of these tumors are invasive.3–6,11 The authors have successfully used intraoperative ultrasound to assist in locating brain tumors and in judging completeness of removal (Figure 30-8). The authors do not recommend pursuing surgical removal of canine meningiomas if adjunctive therapy (e.g., radiation) is not planned to be used following removal. Some gliomas are surgically accessible, but surgical removal/debulking of gliomas is considerably more difficult than meningiomas. Gliomas tend to infiltrate normal brain parenchyma, and it is often difficult to discern tumor margin from brain tissue at surgery. Surgical removal of intracranial glioma is seldom attempted in dogs and cats because of the invasive nature of these neoplasms. Removal of other brain tumor types (e.g., choroid plexus tumor, ependymoma) is also uncommonly performed because of the intraaxial location of these masses. Surgical removal of secondary brain tumors is also not commonly performed because many of these tumors are invasive as are their extraneural source neoplasms. However, some calvarial tumors (e.g., multilobular osteochondrosarcoma [MLO]) are surgically resectable with good outcomes (see Chapter 24).5,18 On rare occasions, metastatic brain tumors are removed surgically. Radiation therapy (RT) is used in the treatment of primary brain tumors, as well as secondary brain tumors that arise by local extension or from metastasis from a distant site. Treatment options have advanced from early reports of the use of orthovoltage RT to current options, including intensity-modulated RT (IMRT) and stereotactic RT (SRT).19,20 Fractionated radiation protocols have largely transitioned from alternate day treatments to daily therapy (Monday through Friday), although there are reports on the application of hypofractionated protocols.21–23 Radiation dose per fraction, total dose of radiation, and the volume of the brain irradiated are important considerations when irradiating brain tumors.24,25 In one report of hypofractionated brain irradiation, 14.5% (12/83) of dogs died or were euthanized due to suspected delayed radiation side effects caused by delivery of a high dose per fraction.22 There are reports on the results of RT alone or in combination with surgery for canine primary brain tumors.19,24,26–29 RT improves survival over surgery alone or medical management alone.26,28 Median overall survival for 29 dogs (presumptive diagnosis of meningioma in 22 dogs, glioma in 4 dogs, and choroid plexus tumor in 3 dogs) that were treated with RT alone was 250 days, with 76% dying of presumptive recurrence or tumor progression.27 In another report of 46 dogs irradiated only for a range of different tumor types the median survival time (MST) was 699 days.24 It is a well-accepted principle that postoperative RT in the microscopic disease setting is generally more efficacious than treating macroscopic disease, including brain tumors. However, it may be difficult to determine if there are postoperative changes versus residual macroscopic disease when CT imaging is done for radiation treatment planning postoperatively.30 With no therapy or only supportive therapy, survival is reported to be 0.2 months in comparison to dogs irradiated with Cobalt-60 external-beam RT alone or in combination with other modalities with a median survival of 4.9 months.26 In another report comparing surgery alone to surgery plus RT, the median survival was 7 months (surgery alone and survival >1 week) compared to 16.5 months (combination therapy).28 In a report of 20 dogs with meningioma that had RT after incomplete resection, the median progression-free survival time was 35 months.29 In a retrospective study of endoscopic-assisted tumor removal in dogs (n = 33) and cats (n = 6), 44% were alive after more than 2 years, only 4 were irradiated postoperatively, and the MST for dogs with meningiomas was 2104 days.31 An alternative to conventional fractionated RT is stereotactic radiosurgery for the management of CNS tumors, which entails the precise delivery of a larger dose per fraction to the tumor in 1 to a few fractions for a total dose of 10 to 15 Gy.20 Limited information is available in the literature, but there are a number of veterinary facilities that now have equipment with this capability. RT has also been used successfully to irradiate a calvarial allograft used in the repair of a calvarial defect in a cat with an intracranial meningioma.32 It is important to consider the potential impact on radiation dose distribution in patients that have undergone surgery and require reconstruction that may include the use of metal implants.33–36 This is potentially of more concern when larger metal implants (e.g., plates, rods, screws) are used to stabilize the spine.37 There are a limited number of reports on the use and efficacy of chemotherapy in the management of brain tumors in companion animals, including individual case reports and small case series.21,38–44 Lomustine, carmustine, and hydroxyurea have been utilized in the treatment of canine brain tumors, but there are limited data to provide validation of the utility of chemotherapy. Based on a retrospective study of oral hydroxyurea in combination with glucocorticoids, 33 dogs with MRI-diagnosed meningioma had a significantly longer survival time than observed in 10 dogs treated with glucocorticoids alone.45 The MST for the combination therapy group was 28 weeks versus 14 weeks for dogs treated with glucocorticoids alone.45 A report on the use of 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) in 206 dogs with a number of different tumor types, including 11 dogs with brain tumors, documented toxicity of this chemotherapeutic agent but did not provide response data.38 Chemotherapy (carmustine, lomustine) alone or in combination with RT or cytoreductive surgery in dogs with astrocytomas has resulted in survival times of 3 to 8 months.21,40 There is limited information on other therapeutic approaches to the management of canine brain tumors, including the use of whole body hyperthermia in conjunction with RT and/or chemotherapy, radioactive iodine-125 implants in combination with other therapeutic approaches, gene therapy, and convection-enhanced delivery of liposomal nanoparticles containing topoisomerase inhibitor CPT-11, which is still being developed.26,46–52 The use of whole body hyperthermia in conjunction with external-beam irradiation did not alter survival.46 Dogs with meningiomas that were irradiated had a mean survival of 314 days versus 288 days for dogs treated with RT plus whole body hyperthermia.46 Gene therapy strategies in dogs have included the delivery of an adeno-associated viral vector containing prodrug activating genes that confer sensitivity to toxic metabolites and cytokine-based gene therapy using an intravenously delivered cationic DNA lipid complex containing murine endostatin gene in a DNA plasmid to perturb tumor angiogenesis.47,48 There is limited information on the application of brachytherapy in conjunction with excisional biopsy, but it may represent another potential avenue of treatment.26,53 Based on the association between vascular endothelial growth factor (VEGF) expression and survival in dogs with intracranial meningiomas, administration of antiangiogenic agents may have future therapeutic potential.54,55 There is information available that indicates that dynamic contrast-enhanced CT may have utility in evaluating and classifying intracranial lesions and for determining response to therapy.56 Prognosis is poor with no therapy or only supportive therapy or palliative therapy in dogs with a MST of 0.2, or less than 2 months.23,26 Results of RT based on a subset of the reports in the literature are difficult to interpret because a definitive histopathologic diagnosis is not always obtained and tumor types are grouped together (including pituitary tumors).19,24,26,27 A presumptive diagnosis may be made based on either CT or MRI findings.27,57 However, this may not be a reliable means of arriving at a presumptive diagnosis.58 The interpretation of results is improving with the advent of minimally invasive techniques for brain tumor biopsies or cytology, as well as advancements in surgical techniques and tumor extirpation for histopathologic analysis, including endoscopic-assisted intracranial tumor removal.31,59–64 The ability to definitively diagnose the specific histopathologic type of brain tumor prior to surgery may aid both clinicians and clients in deciding on a course of treatment and in understanding the potential prognosis.48 Additional potential positive prognostic factors that have been identified include solitary versus multiple lesions, limited neurologic dysfunction, and treatment with RT or a combination of surgery and RT.26,28 Tumor location may also impact survival; MST for forebrain versus caudal brain meningiomas was 2104 days and 702 days, respectively.31 Pneumonia can develop due to aspiration postoperatively and may have a negative impact on survival.65 Meningiomas are the most common intracranial tumor in dogs, but attempts to correlate tumor grade or histologic subtype and MRI features largely have not identified any significant associations that would allow preoperative prognostication.11 In a study of 112 dogs with intracranial meningiomas using criteria of the human WHO international histologic classification system 56% were classified as benign, 43% were atypical, and 1% were malignant meningiomas; the atypical and malignant meningiomas were noted to behave more aggressively with a higher potential for recurrence.11 In one report of 17 dogs treated surgically for meningioma, survival was correlated with histologic tumor type: anaplastic 0 days, fibroblastic 10 days, psammomatous more than 313 days, meningothelial more than 523 days, and transitional 1254 days.44 Of interest is that use of a surgical aspirator to resect intracranial meningiomas resulted in a MST of 1254 days.44 None of the dogs were irradiated, and only 2 of 17 received postoperative chemotherapy (hydroxyurea) when there were MRI findings suggestive of recurrence.44 In a study of 17 dogs with benign meningiomas treated with surgery and postoperative hypofractionated RT, significantly shorter survival times were associated with greater VEGF expression.54 The MST was 748 days for dogs with tumors with 75% or fewer cells staining for VEGF compared with 442.5 days for dogs with tumors with more than 75% of cell staining for VEGF.54 Furthermore, dogs with more intense staining had a significantly shorter survival time.54 In a more recent study, tumor proliferation defined by reactivity of the monoclonal antibody MIB-1 to the Ki67 antigen was not associated with survival, and there was no association between VEGF and tumor proliferation.66 In this study, 70 dogs underwent surgery and postoperative hypofractionated RT, and the overall MST from the date of diagnosis was 514 days.66 Progesterone and estrogen receptor expression has been evaluated in meningiomas in dogs and cats; further investigation will be necessary to determine if there is prognostic significance or a role for targeted intervention.29,67 In one study, progesterone receptor expression was inversely related to tumor proliferative fraction measured by immunohistochemical detection of proliferating cell nuclear antigen (PFPCNA index), which was predictive of survival in dogs with meningiomas after surgery and postoperative RT.29 The 2-year progression-free survival was 43% for tumors with a high PFPCNA index and 91% for tumors with a low PFPCNA index. Furthermore, tumors with a high PFPCNA index were 9.1 times more likely to recur.29 Cyclooxygenase-2 (COX-2) expression has been demonstrated in the majority of intracranial meningiomas (21/24 or 87%), but the role in tumorigenesis and the potential for therapeutic intervention with COX-2 inhibitors have not been elucidated.68 Conversely, in a study of 20 canine gliomas, none of the tumors expressed COX-2.69 In this same study that also evaluated c-kit overexpression, the authors speculated that c-kit inhibitors may have an antiangiogenic effect in high-grade tumors due to presence of intramural vascular expression.69 Stereotactic radiosurgery may provide comparable or improved tumor control with a fewer number of anesthetic episodes but is limited in its application due to a limited number of facilities with this capability.20 Two dogs with meningiomas survived 227 and 56 weeks after radiosurgery; a dog with an oligodendroglioma survived 66 weeks.20 Additional information should be forthcoming with increased availability of RT units with advanced capability to deliver radiation. In dogs with choroid plexus tumors, it should be noted that local spread of the tumor within the ventricular system can occur and distant metastasis via the subarachnoid space occurs in approximately 50% of dogs.13 Additionally, at necropsy in one study of 56 dogs with choroid plexus tumors, 19% had evidence of gross and/or microscopic spinal cord metastases that should impact considerations of diagnostics, approaches to treatment, and prognosis.13 There are descriptive reports but limited prognostic information available for other specific tumor types, such as for astrocytomas.12,70,71 In a report of 86 dogs treated for brain tumors that included 27 meningiomas, 7 astrocytomas, and 6 choroid plexus tumors, survival based on tumor type was evaluated as meningioma versus other; dogs with meningiomas lived significantly longer.26 In a study of dogs irradiated with a hypofractionated protocol, the MST for 34 dogs with intraaxial tumors (presumably gliomas) was 40.4 weeks versus 49.7 weeks for 41 dogs with extraaxial tumors (presumably meningioma, including schwannoma and choroid plexus tumor).22 Histiocytic sarcoma can affect the CNS in dogs with systemic disease, but there are a limited number of reports of localized CNS histiocytic sarcoma, with one report with necropsy information that confirmed the localized nature.72–76 Rapid disease progression and euthanasia was the outcome for two dogs: one with primary spinal cord involvement and the other with brain involvement.76 Surgical intervention has been reported in 12 dogs with subdural histiocytic sarcomas, although no follow-up information was provided.75 There are a number of studies that have been conducted to further elucidate the mechanisms of tumorigenesis and determination of the immunohistochemical profiles of brain tumors.77–83 Such efforts may ultimately lead to a better understanding and ability to prognosticate for individual patients based on identification of specific markers and for determination of appropriate targeted therapy. However, to date, targeted therapies in humans with meningiomas have only realized modest success and are associated with toxicity.84 It should be noted that multiple meningiomas, multifocal oligodendroglioma, and synchronous brain tumors (oligodendroglioma and meningioma) have been reported, as well as synchronous primary and metastatic tumors in dogs.43,85–89 Based on one small case series, survival may be comparable for dogs with multiple meningiomas as seen for dogs treated for a solitary meningioma.43 Additionally, dogs with a primary brain tumor may have another primary tumor that may affect prognosis.7 In a report of 170 dogs with primary intracranial neoplasia, 38/170 (23%) had another tumor unrelated to the brain tumor.7 In a study of 28 dogs with meningiomas 7 (25%) had another tumor in addition to the meningioma.87 Although rare, pulmonary metastasis in three dogs with intracranial meningioma has been reported.90 Cats are also reported to have multiple or two different types of intracranial tumors and, in fact, more commonly have multiple meningiomas (17%) than dogs.2,8,91,92 It has been questioned whether multiple meningiomas represent multicentric disease or metastasis. Dogs with metastatic intracranial neoplasms most commonly have metastatic hemangiosarcoma or carcinomas, representing 50% and 20% of metastatic tumors, respectively, with an anticipated poor prognosis.10,93 Meningeal carcinomatosis results from spread of a solid tumor that can be an intracranial primary tumor or of distant origin (e.g., mammary gland carcinoma) and is associated with a very poor prognosis.94–96 Metastatic disease is usually recognized as multiple mass lesions, but a solitary lesion may represent metastasis. Full staging is necessary to rule out other disease because secondary intracranial neoplasia may be more common than primary intracranial neoplasia.10 Cats respond well to surgery alone for intracranial meningiomas, which is the most common primary intracranial tumor in cats, and have a MST of approximately 2 years.2,9,97,98 In one report, the MST for cats treated surgically was 685 days compared to 18 days for cats that did not have surgery.2 Recurrence is documented to occur; 20.6% in one report had a median postoperative time to recurrence of 285 days (range 123 to 683 days; n = 6 cats); second surgeries are feasible in cats.2,9 Cats with multiple meningiomas based on one report have long-term survival when treated surgically with follow-up chemotherapy (hydroxyurea) for 2 (n = 3 cats) or 4 (n = 1 cat) meningiomas.91 Cats with intracranial lymphoma more commonly have multicentric disease; fewer cats have primary intracranial lymphoma.2 In cats treated palliatively with systemic corticosteroids, the MST was 21 days (range 9 to 270 days in nine cats).2 Two cats with oligodendrogliomas were euthanized at the time of diagnosis or 6 weeks after diagnosis due to their poor neurologic status.99 Of six cats with oligodendrogliomas, three had surgery (n = 2) and/or received corticosteroids (n = 1), with survival times of 1 day, 5 days, and one cat that had surgery was lost to follow-up at 1 month.2 Four cats with cerebral astrocytomas (three of which were managed medically) lived for 1 to 3 years with anticonvulsant therapy.100 Two of six cats with astrocytomas had surgery, followed by RT in one cat with survival times of 1 day and 179 days; a third cat treated with only corticosteroids survived 35 days.2 One cat with an ependymoma that had two surgeries ultimately survived for 667 days; another cat treated with corticosteroids was euthanized at 685 days.2
Tumors of the Nervous System
Brain Tumors
Pathology
History and Clinical Signs
Diagnostic Techniques and Work-Up
Imaging
Cerebrospinal Fluid Analysis
Therapy
Radiation Therapy
Chemotherapy
Other Therapies
Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine