Chapter 24. Tumors of the Musculoskeletal System
David Ruslander
Tumors of the musculoskeletal system are relatively rare in dogs and cats with the exception of osteosarcoma (OSA), which accounts for the vast majority of primary skeletal tumors in dogs. This chapter covers primary and metastatic tumors, but osseous manifestations of other tumors including multiple myeloma, plasmacytoma, and lymphoma are covered in separate chapters.
PRIMARY APPENDICULAR SKELETAL TUMORS
Incidence and Etiology
Osteosarcoma is the most common primary bone tumor in dogs, accounting for approximately 5% of all neoplasia and the overwhelming majority of canine bone tumors. 1-3 Other tumors of bone include, but are not limited to, chondrosarcoma (CSA), fibrosarcoma (FSA), hemangiosarcoma (HSA), and histiocytic sarcoma. Synovial cell sarcoma is a tumor of the joint capsule and not a classic bone tumor, but has similar clinical features to the skeletal tumors. Digital tumors such as squamous cell carcinoma (SCC) and malignant melanoma, although not classical musculoskeletal tumors, also affect the bones of the digit and need to be considered here (additional information is available in Chapter 23, Section A ). The overall incidence of canine OSA is low in the general population but quite high in certain breed groups. The incidence is much lower in cats. Classically, OSA affects the long bones, but can be seen in any bone of the appendicular and axial skeleton including unusual sites such as the os penis. Appendicular OSA occurs in large breed dogs in classic locations (see later discussion), but axial OSA can be seen in any breed in any location. 4-6 The exact etiology is unknown, but it has been theorized that microfractures in the weight-bearing portion of the bone lead to malignant transformation of the osteoblasts. 7 Metallic implants, ionizing radiation (resulting from therapeutic radiation), and bone infection (osteomyelitis) have also been reported as potential risk factors for development of OSA. 8-16 Height of the dog, rather than weight, has also been shown to be a risk factor. 17 Neutering status has been implicated as a risk factor, and in one study Rottweilers that were spayed or neutered prior to 12 months of age had a 4× increased risk for the development of OSA. 18 Extraskeletal OSA has been reported in both species, can include cutaneous and non-cutaneous locations, and is associated with a poor prognosis. 19-21
Genetic factors appear to play a role in the development of OSA, given the increased risk of OSA in specific breeds. Recently, a host of genes have been shown to be differentially expressed in dogs with OSA including the tumor suppressor genes p53, retinoblastoma (Rb) and insulin-like growth factor (IGF-1), PTEN, c-met, Ezrin, erbB-2, and possibly c-kit. 22-33 Cyclooxygenase-2 (COX-2) expression has also been identified in OSA tissue in dogs. 34 Expression and mutation of these and other genes have varying levels of prognostic significance for OSA. Molecular aspects of feline OSA are poorly described.
Multicentric digital SCC is a recognized syndrome in which dogs appear with multiple digit involvement, without metastatic disease. Labrador retrievers, giant schnauzers, and standard poodles are breeds that appear to be overrepresented for this poorly understood disease process, suggesting a genetic predisposition. 35
Clinical Features
Most dogs with primary bone neoplasia appear to be pain because of bone destruction. Many are presented with swelling of the affected limb, but acute lameness may be all that is apparent, especially with proximal bone lesions. Advanced cases may be presented with an acute pathologic fracture. Axial lesions in the oral cavity, spine, and ribs may be more chronic in nature, and clinical signs more subtle. Cats generally are presented with lameness or swelling.
Diagnosis in many situations is delayed by failure to recognize the disease entity and a tendency to initially treat with non-specific pain management and forego radiographs. Ideally, all large breed dogs with significant or persistent lameness should be evaluated with multiple-view radiographs of the affected limb at presentation. Long-standing discomfort in patients can lead to systemic signs such as weight loss and anorexia, but this obviously should be differentiated from metastatic disease or concurrent disease.
Diagnosis and Staging
Patients presenting for evaluation of any suspected bone neoplasia should be properly staged before initiation of therapy. Radiographs of the affected limb are often diagnostic when the lesion is in a classic location and demonstrates the “sunburst” appearance of simultaneous proliferation and destruction of bone ( Figure 24-1 ) that does not cross the joint. This is in contrast to synovial cell sarcoma, which will produce radiographic changes on both sides of the affected joint. Some lesions can be more proliferative, whereas others are more lytic. Differentials for such radiographic findings are limited to bone neoplasia, bacterial or fungal osteomyelitis, bone cysts, and healing bone injury. A predominance of lysis without proliferation should alert the clinician to the possibility of alternative diagnoses, including metastatic carcinoma. However, primarily lytic lesions may be a feature of OSA, especially in greyhounds, which are more likely to appear with a pathological fracture. Location of the lesion in the bone is often helpful, since most primary bone tumors are located in the metaphyseal region whereas metastatic lesions are often diaphyseal. The vast majority of primary OSAs are seen in the following locations: distal radius, proximal humerus, proximal tibia, and distal femur. Other sites including distal tibia, distal humerus, ulna. and proximal femur are less commonly affected. Three-view chest radiographs should be evaluated before any biopsy or surgical intervention. Non-OSA neoplasia may have a higher rate of nodal and systemic metastasis, especially histiocytic neoplasia, so lymph node aspiration should be considered in patients with suspected non-OSA (non-classic breed, location, or cytologic finding) even if the regional lymph node is not enlarged. The incidence of overtly metastatic disease is low in OSA (approximately 6%) at diagnosis, but should it exist, prognosis and treatment may be dramatically altered. 36 CT scanning may help identify early subtle lesions in the lungs. Abdominal ultrasound is indicated when bone lesions are suspected to be metastatic. Otherwise, abdominal ultrasound is not routinely used unless clinical signs or the biochemical profile is abnormal. Nuclear imaging (bone scan) can be useful in determining the extent of the bone lesions and to determine if the patient has metastasis to other bones prior to surgical intervention. 37
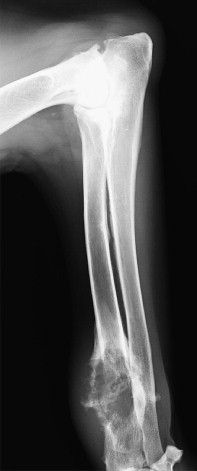 |
| FIGURE 24-1 Lateral radiograph of a 9-year-old St. Bernard showing classic radiographic appearance of an osteosarcoma. Note the distal radius location and both osteolytic and osteoproductive lesions. |
Although bone biopsy facilitates histologic confirmation, diagnosis can often be made via fine needle aspiration under mild sedation. Differentiation between OSA and other bone neoplasia on cytology may be difficult short of special staining for alkaline phosphatase; however, this is often not necessary if the location and breed is consistent with OSA. 38-43 Histopathologic confirmation may be needed in situations in which the cytology is non-diagnostic or the owner wants confirmation or additional prognostic information before making therapy decisions. 44 Owners should be warned about false-negative biopsy results, which can occur in 10% to 20% of cases. Bone biopsy (described in Chapter 6 ) can be accomplished with an open or closed approach using either a Jamshidi needle or Michelle trephine instrument. The latter is more aggressive and more likely to cause iatrogenic pathologic fracture or other complications, although the risk of fracture is probably overstated. Care should be taken when choosing the biopsy site so that contaminated tissue can be excised at the time of limb-sparing surgery if this option is being considered.
Biological Behavior
OSA is characterized by aggressive local bone destruction and high likelihood (>90%) of eventual metastasis, although fewer than 10% of affected dogs have overt metastatic disease at presentation. 45 The concept of micrometastatic disease is difficult for owners to Comprehend, but it is critical to understanding the disease process since most dogs eventually will succumb to metastatic disease even after local control of the tumor is accomplished via amputation or limb-sparing procedures. Early metastatic lesions are below the level of detection with conventional imaging such as radiographs or even CT scans, but likely will grow over the ensuing months to years following diagnosis. It has been suggested that the metastatic rate of axial OSA may be lower than appendicular disease, but this may be a manifestation of poor local disease control with tumors in the oral cavity, ribs, or vertebrae, since many of these patients succumb to local recurrence of the tumor before the development of metastatic disease. 46-49 CSA, while seen primarily in the nasal cavity and other flat bones, can also occur in the appendicular skeleton and tends to be less metastatic than OSA, although this appears to be dependent upon grade of the tumor. 50,51 FSA of the bone is extremely rare in dogs and may be cured with surgery alone. The metastatic rate appears to be low but may be influenced by mitotic rate and grade of tumor, similar to soft tissue sarcomas. Synovial cell sarcomas are locally aggressive and have the potential to metastasize, depending on the grade of the tumor. 52,53 HSA and histiocytic sarcoma (previously known as malignant histiocytosis) of the bone may be primary or metastatic tumors and are considered highly malignant and highly metastatic even if no distant disease is noted at presentation. Multiple synchronous metastatic lesions may occur, affecting bone and other sites such as skin or internal organs.
Digital neoplasia has a moderate rate of metastasis. Accordingly, lymph node aspiration and chest radiographs are recommended. 54-57 In addition, abdominal ultrasound is advised for patients with hind limb digits affected.
Treatment Options ( Table 24-1 )
Surgery
Treatment of bone neoplasia is both local and systemic in nature, especially for OSA, HSA, and histiocytic sarcoma. Surgical options include amputation or limb-sparing procedures. Although effective at removing the source of pain, amputation by itself does not appear to prolong survival in OSA (other than preventing pain-related death) but may be curative for CSA or FSA. Amputation of the affected limb is well tolerated, assuming the patient does not have severe concurrent musculoskeletal or neurologic conditions. Most properly screened dogs tolerate the procedure very well, and owner satisfaction with amputation is excellent. 58 Tumors located in the scapula can be surgically removed via a partial or total scapulectomy, not necessitating limb amputation. 59,60
| Treatment | Details | Median Survival | 1-Year Survival | 2-Year Survival | No. of Cases | Author |
|---|---|---|---|---|---|---|
| Amputation Alone | ||||||
| 19.2 wks | 11.5% | 2.0% | 162 | Spodnick 45 | ||
| 175 days | 21% | 0% | 19 | Mauldin 88 | ||
| 14.5 wks | 0% | 0% | 8 | Shapiro 89 | ||
| 119 days | 11% | 4% | 35 | Straw 91 | ||
| 168 days | 20% | 0% | 15 | Thompson 93 | ||
| Amputation and Chemotherapy | ||||||
| Cisplatin × 2 | ~272 days | 38% | 17% | 36 | Straw 91 | |
| Cisplatin/doxorubicin × 2 (alt) | 300 days | 36% | 26% | 19 | Mauldin 88 | |
| Cisplatin × 2-6 | 43 weeks | 30% | 10% | 11 | Shapiro 89 | |
| Cisplatin × 1-6 (some had limb sparing) | 46.4 weeks | 45.5% | 20.9% | 22 | Berg 61 | |
| Cisplatin × 6 | 413 days | 62% | 19% | 16 | Kragel 90 | |
| Cisplatin × 2 before or after Sx | 262/282 days | 38%/43% | 18%/16% | 19 | Straw 91 | |
| Cisplatin × 2 | 290 days | 33.3% | 6.6% | 15 | Thompson 93 | |
| Doxorubicin (2 or 3 pre-Sx) | 52.3 weeks | 50.5% | 9.7% | 35 | Berg 94 | |
| Carboplatin/Doxorubicin (concurrent) | 235 days | 8.3% | 4.1% | 24 | Bailey 101 | |
| Carboplatin × 3 (1 pre-SX) | 230 days | 30% | 30% | 21 | Khanna 98 | |
| Cisplatin/Doxorubicin concurrent × 4 | 300 days | 28.5% | 5% | 35 | Chun 104 | |
| Lobaplatin × 4 | ? | 31.8% | ? | 28 | Kirpensteijn 99 | |
| Liposome Cisplatin | 333 days | 50% | 40%/0% | 20 | Vail 100 | |
| Carboplatin × 4 (1 pre-Sx) | 207 days | 25% | 20% | 18 | Vail 100 | |
| Carboplatin × 4 (1 pre-Sx) | 321 days | 35.4% | 48 | Bergman 95 | ||
| Cisplatin/Doxorubicin concurrent (2 or 10 days post-Sx) × 3 | 11.5 months | 47% | 28% | 102 | Berg 96 | |
| Carboplatin/Doxorubicin alternating × 3 | 227 days | 48% | 18% | 32 | Kent 103 | |
| Limb-Sparing Surgery | ||||||
| Misc protocols | 8 months | ? | ? | 20 | Larue 62 | |
| No chemotherapy | Thrall 66 | |||||
| Intraarterial Cisplatin ± RT | 49 | Withrow 79 | ||||
| Extra Corporeal RT (70Gy) + OPLA-PT + chemo | 298 days | 23% | 15% | 13 | Liptak 80 | |
| OPLA – PT + Carbo or Carbo/Doxorubicin | 429 days | NA | NA | 47 | Lascelles 69 | |
| Humerus ± chemotherapy | 172 days | ? | ? | 17 | Kuntz 68 | |
| Radiation Therapy | ||||||
| Palliative 10 Gy × 3 (0.7.21 day) | 125 days | 6.6% | 0% | 15 | McEntee 76 | |
| Axial Palliative (20–30 Gy)/Curative (45–57 Gy) | 137 day (79 Palliative vs. 265 Curative) | 22 | Dickerson 71 | |||
| Curative intent (48–59.4 Gy) ± OPLA-Pt + chemotherapy | 209 days | 21% | 7.1% | 14 | Walters 72 | |
| Stereotactic RT (20–30 Gy) | 363 days | 27.2% | 9.0% | 11 | Farese 73 | |
| Palliative 8 Gy × 4 weekly + chemotherapy | 313/162 days (appendicular/axial) | 20.8% | 0% | 24 | Green 74 | |
| Intra-arterial Cisplatin + RT | 4.9 months | 25% | 12 | Heidner 75 | ||
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


