Chapter 25. Tumors of the Hematopoietic System
SECTION A Lymphoma
Jeffrey N. Bryan
LYMPHOMA IN DOGS
Incidence/Etiology and Risk Factors
Lymphoma is a collection of neoplasms arising from the malignant transformation of lymphocytes. Incidence in dogs increases with increasing age, leveling off somewhat after 6 years. 1 Lymphoma can appear in dogs of any age, breed, or sex, although certain breeds ( Table 25-1 ) and intact females (relative risk of 0.7) appear to have a lower incidence of the disease. 1 The development of lymphoma has been associated with exposure to the 2,4D herbicide, although an industry re-examination of these data weakened the initial association. 2-4 Industrial living and chemical use by owners have been associated with development of lymphoma. 5 The disease has also been demonstrated to be genetically heritable in families of dogs. 6 Immunosuppressive medications such as cyclosporine increase the risk of lymphoma in humans and likely cats, suggesting that this may be true in dogs as well. 7 Genomic loss of methylation of cytosine bases in the canine chromosomes has also been associated with lymphoma. 8
| Breed | Relative Risk 1 |
|---|---|
| Increased Risk | |
| Boxer | 4.5 |
| Basset Hound | 4.1 |
| St. Bernard | 4.0 |
| Scottish Terrier | 3.2 |
| Airedale | 2.7 |
| Labrador Retriever | 1.8 |
| Bulldog | 2.2 |
| Lower Risk | |
| Dachshund | 0.1 |
| Pomeranian | 0.1 |
| Pekingese | 0.3 |
| Miniature/Toy Poodle | 0.3 |
| Chihuahua | 0.2 |
| Brittany Spaniel | 0.1 |
Signalment and Clinical Features
Presentation can be indolent or aggressive. Lymphoma can be solitary or multicentric, node based or associated with any organ in the body. Early, accurate diagnosis and careful staging are keys to proper clinical decision making. Dogs typically present with non-painful lymphadenomegaly. Tumor stage has been shown to be prognostic ( Table 25-2 ), and staging tests should be performed routinely. Prognostic variables are listed ( Table 25-3 ). Canine lymphoma is generally the non-Hodgkin’s form of the disease, although Hodgkin’s lymphoma has been reported. 9-11
| Stage | Characteristics |
|---|---|
| I | 1 lymph node affected |
| II | >1 lymph node affected, same side of diaphragm |
| III | Multiple nodes, both sides of diaphragm |
| IV | Liver or spleen involvement +/- I-III |
| V | Marrow or extranodal tissue involvement +/- I-III |
| Substage | |
| A | No clinical signs referable to lymphoma |
| B | Clinical signs referable to lymphoma |
| Prognostic Variable | Critical Factor | Prognosis |
|---|---|---|
| WHO stage | Stage I/II | + |
| Stage III–V | − | |
| WHO substage | b | − |
| Histologic subtype | Low grade | Longer survival, lower chemoresponsiveness |
| High grade | Higher chemoresponsiveness, shorter survival | |
| Immunophenotype | T cell | − |
| Hypercalcemia | Elevated iCa | − |
| Sex | Female | + |
| P-glycoprotein expression | Present | − |
| Prednisone prior to chemotherapy | If prolonged exposure | − |
| Mediastinal location | Present | − |
| Extranodal location | GI, renal, or cutaneous | − |
| Trisomy 13 | Present | + |
Diagnosis and Staging
A diagnosis of lymphoma is most commonly established using cytology or biopsy. Cytology allows rapid identification of a monomorphic, abnormal lymphocyte population, but does not give complete classification and grading information ( Figure 25-1 ). Histopathologic examination allows complete classification and grading, as well as immunophenotyping of the disease, yielding prognostic information. Classification systems in use include the National Institutes of Health Working Formulation and the Kiel system. 9,10,12 Both systems evaluate architecture and cellular morphology; the former does not include immunophenotype, but the latter includes immunophenotype in classification. Low-grade lymphomas, including mantle-zone, marginal-zone, follicular, and T-zone origins, have been associated with a significantly better survival prognosis than intermediate- and high-grade lymphomas. 13 Recently, flow-cytometry and immunoglobulin-gene and T-cell receptor gene rearrangement have been used to diagnose and type lymphomas. 14-24 Cytogenetics have also been used to identify chromosomal abnormalities in canine lymphomas. 25 Many extranodal locations have been reported ( Table 25-4 ). Complete staging is critical for effective therapeutic decision-making ( Table 25-5 ). Positron emission tomography (PET) imaging may offer an extremely sensitive staging in a single examination in the future. 26 Thorough patient evaluation allows the detection of prognostic factors that may have bearing on a client’s decision to treat, and allow careful staging of induction with chemotherapy to minimize patient morbidity through toxicity.
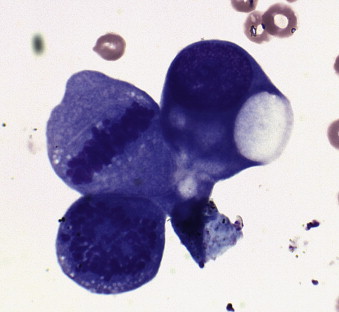 |
| FIGURE 25-1 Photomicrograph of a lymph node aspirate cytology preparation. Note the large cellular size relative to the red blood cell. The lymphoma cells display anisokaryosis as well as large and irregular nucleoli, and at least one mitotic figure is visible. |
| Location | Canine Reference | Feline Reference | Comments |
|---|---|---|---|
| Cardiac | 27 | 28 | |
| Hepatosplenic | 29,30 | ||
| Ocular/orbital | 31 | ||
| Conjunctiva | 32,33 | 34 | |
| Synovial | 35 | ||
| Cutaneous | 36–38 | 39–41 | |
| Intestinal | 42,43 | 44–52 | |
| Hypercalcemia | 53 | 54,55 | T-cell lymphoma |
| Skeletal muscle | 56,57 | 58 | |
| Bone | 59 | ||
| Brachial plexus | 60 | ||
| Spinal cord | 61,62 | ||
| Brain | 63 | ||
| Tympanic bulla | 64 | ||
| Hypereosinophilia | 43 | 47 | T-cell lymphoma |
| Intravascular | 65–68 | 69 | |
| Upper respiratory | 70,71 | 72–74 | |
| Trachea | 75 | ||
| Lung | 58 | ||
| Kidney | 76 | 77 | |
| Urinary bladder | 78 | ||
| Urethra | 79 | ||
| Myasthenia gravis | 80 |
| Staging Tests | Purpose | Stage If Positive |
|---|---|---|
| Lymph node aspirate | Rapid diagnosis | I–V |
| CBC | Detect cytopenias or anemia that complicate treatment or the presence of leukemia | V |
| Chemistry profile | Detect organ dysfunctions that complicate treatment/indicate organ involvement | IV or V possible |
| Urinalysis | Detect complicating urinary tract disease or renal compromise | V possible |
| Lymph node biopsy | Establish tumor grade and immunophenotype | I–V |
| Thoracic radiographs | Detect thoracic node or pulmonary involvement | V if pulmonary lymphoma |
| Abdominal ultrasound | Detect organ or abdominal node involvement | IV if liver/spleen lymphoma |
| Bone marrow aspirate | Detect bone marrow compromise | V if lymphoma cells present |
| Ophthalmic examination | Detect ocular involvement | V if lymphoma present |
Biological Behavior/Metastasis
Lymphoma is most commonly a systemic illness and is assumed to be metastatic at presentation. Localized lymphomas have been reported, but systemic therapy is nearly always warranted for effective management. Multicentric lymphoma is the most common presentation. This disease appears to arise in one node or group of nodes and progress to involve many nodes, any lymphoid tissue, and even non-lymphoid tissue, as reflected by the World Health Organization (WHO) staging scheme. Alimentary lymphoma occurs less frequently than multicentric lymphoma. This form of lymphoma may be associated with significant hypoproteinemia, which has been identified as a negative prognostic factor for survival. Alimentary lymphoma must be differentiated from lymphocytic/plasmacytic enteritis. Chronic lymphocytic enteritis has been suggested to precede the development of alimentary lymphoma, although this has not been definitively demonstrated. A reported hallmark of lymphoma is invasion of the muscularis of the intestine, ruling out lymphocytic enteritis. Early literature suggested B-cell origin for the majority of these tumors, but recent reports suggest that T-cell origin may be frequent and associated with an eosinophilic infiltrate. 42,43,81,82 Central nervous system (CNS) lymphoma can occur as the primary presentation, or lymphoma may affect the CNS as a secondary site. CNS infiltration may be focal or diffuse (see Chapter 19 ). The cutaneous form of lyphoma is most classically represented by epitheliotropic T-cell lymphoma (mycosis fungoides), although B-cell cutaneous lymphoma has been reported. This form of the disease is progressive and usually becomes systemic in its course. Renal lymphoma in dogs has been associated with a poor prognosis, but case reports exist of long-term survival. 76
Therapy and Prognosis
Chemotherapy
The mainstay of therapy for canine lymphoma is cytotoxic chemotherapy. Numerous protocols have been evaluated in the literature ( Table 25-6 ). Of these, the multi-drug protocols that include doxorubicin, vincristine, cyclophosphamide, and prednisone (CHOP) have met with the greatest success. Given in various combinations, these drugs typically yield greater than 80% complete response, remissions of approximately 9 months, and median survival greater than 1 year when followed by rescue therapy. The Madison-Wisconsin 25-week protocol, reported by Garrett and others, 83 led to a 94% complete response rate with 100% of those patients achieving a second remission after reinduction with the same protocol ( Table 25-7 ). The 25-week version has no maintenance phase, which allows dogs to live for a period after chemotherapy without treatment. This may lead to greater responsiveness after loss of remission by lack of selection for resistance during the new rapid growth phase. Similarly, Moore and others 84 found no loss of effectiveness of disease control by using a discontinuous protocol of VELCAP without a maintenance phase in the initial therapy period. It now appears that there is no survival benefit to a maintenance phase of initial chemotherapy for the average canine patient. Both protocols described include l -asparaginase. MacDonald and others 85 published a follow-up study to the original Wisconsin-Madison 25-week protocol that found no difference in response rate or survival with the omission of the l -asparaginase during induction. This suggests that reserving that drug for rescue may be most appropriate. 85,86 If used, l -asparaginase should be administered intramuscularly. 87 Drug resistance may be mediated by p -glycoprotein encoded by the multi-drug resistance gene (MDR1). 88-91 P -glycoprotein expression has been documented in lymphomas and is likely a negative prognostic factor. 89,90,92 Mutation of this gene may be associated with increased chemotherapy toxicity. 93
| ∗ CHOP-based protocol. | |||
| Chemotherapy Protocol | CR Rate | Median Survival or Response | Reference |
|---|---|---|---|
| COP | 70%–75% | 7.5 mo | 107,108 |
| Madison-Wisconsin 25 wk ∗ | 94% | 13.2 mo | 83 |
| Lomustine alone (cutaneous disease) | 17% | 3.5-mo response | 97 |
| Madison-Wisconsin 25 wk w/Actino | 7 mo | 94 | |
| VELCAP-S ∗ | 94% | 12.5mo overall and 17 mo if CR | 84 |
| VELCAP-SC ∗ | 70% | 6 mo | |
| VELCAP-HDC ∗ | 93% | 32 mo for high-dose cyclo w/transplant | 99 |
| Madison-Wisconsin 25 wk w/Half Body RTX ∗ | 85% | 19 mo if CR | 109 |
| Doxorubicin single agent | 59%–79% | 7.3–9 mo | |
| Mitoxantrone | 25% | 3-mo median remission | 95 |
| Perform a CBC with platelet count before and 7 days after every treatment. | ||
| Vincristine dosage range = 0.5 to 0.7 mg/m 2 . | ||
| ∗ Administer (qs 30 ml in 0.9% NaCl) over 1/2 hour minimum. | ||
| ∗ Doxorubicin dosage decreased to 1 mg/kg in dogs weighing < 10kg. | ||
| Week 1 | l-Asparaginase/Vincristine/Prednisone | 10,000 U/m 2 IM/0.7 mg/m 2 IV/2 mg/kg PO daily |
| Week 2 | Cyclophosphamide/Prednisone | 250 mg/m 2 PO divided over 4 days/1.5 mg/kg PO daily |
| Week 3 | Vincristine/Prednisone | 0.7 mg/m 2 IV/1 mg/kg PO daily |
| Week 4 | Doxorubicin ∗ /Prednisone | 30 mg/m 2 IV/0.5 mg/kg PO daily |
| Week 6 | Vincristine | 0.7 mg/m 2 IV † |
| Week 7 | Cyclophosphamide | 250 mg/m 2 PO divided over 4 days |
| Week 8 | Vincristine | 0.7 mg/m 2 IV |
| Week 9 | Doxorubicin ∗ | 30 mg/m 2 IV |
| Week 11 | Vincristine | 0.7 mg/m 2 IV |
| Week 13 | Cyclophosphamide | 250 mg/m 2 PO divided over 4 days |
| Week 15 | Vincristine | 0.7 mg/m 2 IV |
| Week 17 | Doxorubicin | 30 mg/m 2 IV |
| Week 19 | Vincristine | 0.7 mg/m 2 IV |
| Week 21 | Cyclophosphamide | 250 mg/m 2 PO divided over 4 days |
| Week 23 | Vincristine | 0.7 mg/m 2 IV |
| Week 25 | Doxorubicin | 30 mg/m 2 IV |
A chemotherapy protocol must be selected and tailored to meet the patient’s and client’s needs. The ideal protocol (1) will be within the client’s budget and have a toxicity profile on par with the client’s willingness to assume risk, (2) will avoid drugs with toxic profiles that could target a particular weakness of the patient in question, and (3) will result in remission for the patient. The chronic chemotherapy toxicity of most concern in canine lymphoma patients is the cardiotoxicity associated with doxorubicin. Doxorubicin has been associated with the most durable remissions of the drugs available to treat lymphoma. The chronic cardiotoxicity associated with its administration can be a contraindication to its use. In these patients, the use of dexrazoxane, an iron-free radical scavenger, may prevent damage to an already compromised myocardium. The use of other anthracycline drugs has also been explored. Unfortunately, actinomycin-D as a replacement for doxorubicin resulted in diminished survival in an otherwise identical protocol. 94 Mitoxantrone has also been associated with a lower response rate. 95,96
One of the greatest apparent benefits of chemotherapy protocols without a maintenance phase is the high proportion of patients who will reenter remission when the protocol is repeated following loss of remission after therapy is completed. This simplifies initial rescue therapy decisions. When patients have failed resumption of initial therapy, it is necessary to choose among a series of single drug and combination chemotherapy options, each with toxicity and efficacy differences ( Table 25-8 ). Subsequent remissions tend to be shorter than the initial remission. The MOPP (mechlorethamine, vincristine, procarbazine, prednisone) protocol has been associated with longer second remissions in a subset of patients, however. Subsequent losses of remission portend a worsening prognosis.
| OPP, Vincristine, procarbazine, prednisone. | ||||||
| Rescue Chemotherapy Protocol | No. of Dogs | CR Rate | PR Rate | Median Duration of Response | Notes | Reference |
|---|---|---|---|---|---|---|
| Actinomycin-D | 9 | 33% | 44% | 1.5 mo | 110 | |
| Actinomycin-D | 25 | 0% | 0% | 0 mo | No response observed | 111 |
| Mitoxantrone | 34 | 38% | 26% | 4 mo if CR | Not dissimilar to mitoxantrone first | 95 |
| Mitoxantrone | 15 | 47% | 47% | 3 mo if CR | Much lower than doxorubicin | 96 |
| Doxorubicin | 12 | 42% | 33% | 5 mo | No dog responded to second dose if failed first | 112 |
| Doxorubicin/Dacarbazine | 15 | 53% | 33% | 1 mo | All had received doxorubicin prior | 113 |
| MOPP | 117 | 65% | 31% | 2 mo if CR | 9/76 had longer rescue remission | 114 |
| Cisplatin/cytosine arabinoside | 10 | 30% | 10% | 1.5 mo | Abstract | Ruslander |
| Etoposide | 12 | 15% | 7% | Cutaneous reactions to Polysorbate-80 | 115 | |
| CCNU (Lomustine) | 43 | 27% | 7% | 3 mo overall, 4 mo if CR | 116 | |
| B(Carmustine)OPP | 14 | 29% | 21% | 4 mo if CR | Neutropenia, thrombocytopenia, and GI tox | 117 |
| L(Lomustine)OPP | 40 | 27% | 25% | 4 mo if CR | Neutropenia, thrombocytopenia, and GI tox | 117 |
Clinically ill or Stage V dogs with low neutrophil or platelet counts require cautious initiation of chemotherapy. In these patients, treatment with l -asparaginase and prednisone after complete staging may cause downstaging of the disease without causing myelosuppression. Cytotoxic therapy may be initiated 3 to 5 days later when the animal feels clinically well or the cell counts have returned to normal.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


