Chapter 20. Tumors of the Head and Neck
SECTION A Oral and Salivary Gland Tumors
Carolyn J. Henry and Mary Lynn Higginbotham
Salivary Gland Tumors
• The majority of canine and feline salivary tumors are malignant, with adenocarcinoma the most frequent diagnosis.
• Metastasis at the time of diagnosis is more common in cats than dogs and may develop in the regional lymph nodes and other distant locations late in the course of disease.
• Local recurrence is common with surgical excision alone.
ORAL TUMORS
Incidence—Morbidity and Mortality
Oral tumors account for 3% to 10% of all feline neoplastic diseases and are the fourth most common neoplasm in dogs. 1-3 The most common oral malignancies in dogs are malignant melanoma (MM), squamous cell carcinoma (SCC), and fibrosarcoma (FSA). In cats, SCC predominates, with FSA seen less frequently. The most common tongue tumor diagnosed in cats is SCC, whereas lingual melanomas occur more often in dogs, followed by SCC ( Figure 20-1 ; BOX 20-1 and BOX 20-2 ). 1-14 Benign neoplastic and inflammatory lesions (viral papillomatosis, epulides/ameloblastomas, odontomas, and eosinophilic granulomas) are differential diagnoses to consider for patients presenting with an oral mass.
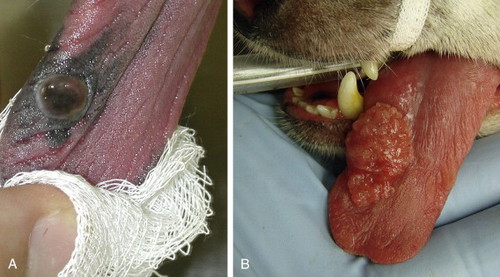 |
| FIGURE 20-1 A, Melanoma and, B, squamous cell carcinoma are the most common lingual tumors in dogs. |
BOX 20-1
REPORTED ORAL MALIGNANCIES IN DOGS AND CATS 1-14
• Melanoma (most common oral malignancy in dogs)
• Squamous cell carcinoma (most common oral malignancy in cats)
• Fibrosarcoma
• Osteosarcoma
• Mast cell tumor
• Extramedullary plasmacytoma
• Lymphoma
• Chondrosarcoma
• Hemangiosarcoma
• Anaplastic sarcoma
• Multilobular osteochondrosarcoma/Multilobular tumor of bone
• Myxosarcoma
• Neurofibrosarcoma
• Rhabdomyosarcoma
• Transmissible venereal tumor
• Ectopic thyroid carcinoma (tongue)
BOX 20-2
TONGUE TUMORS REPORTED IN DOGS AND CATS 7,9-13
Malignant
Squamous cell carcinoma (most common in cats)
Malignant melanoma (most common in dogs)
Fibrosarcoma
Mast cell tumor
Adenocarcinoma
Leiomyosarcoma
Neurofibrosarcoma
Rhabdomyosarcoma
Hemangiosarcoma
Ectopic thyroid carcinoma
Benign
Plasmacytoma
Granular cell myoblastoma
Papilloma
Rhabdomyoma
Lipoma
Hemangioma
Myxoma
Etiology/Risk Factors
Unlike oral cancer in people, which is largely attributable to the use of smokeless tobacco products, the underlying etiology for most oral malignancies in companion animals is unknown. Three notable exceptions are: (1) viral papillomatosis occurs subsequent to horizontal transmission of papovavirus from dog to dog (see Chapter 3, Section B ); (2) radiation-induced oral sarcomas and carcinomas may occur years after radiation therapy for benign oral tumors such as epulides 15,16 ; and (3) an association between exposure to environmental tobacco smoke and development of some feline oral SCC is supported by both epidemiologic and molecular evidence. 17
Clinical Features
Animals with tumors located in the rostral oral cavity are usually presented for evaluation of a visible mass. Rostral tumors are detected earlier and therefore are usually smaller. This means there is a greater likelihood for complete excision; and such tumors often have a good prognosis, especially canine SCC. 3,5,18 Tumors in other sites may go undetected since they are difficult to see or may be ignored because of their benign outward appearance. Oftentimes, suspicion of oral cancer develops during routine dental prophylaxis. One such hallmark of oral neoplasia is the presence of loose teeth in a patient with otherwise good dentition. This presentation warrants tissue biopsy at the time of tooth extraction. FSAs of the maxilla are often associated with considerable facial deformity ( Figure 20-2 ) and may be biologically aggressive, despite a low-grade appearance on histopathology. Other typical clinical signs that should prompt a thorough oral examination are listed in Box 20-3 . The presence of multiple concurrent oral lesions is most consistent with benign conditions, including papillomas and epulides, but does not rule out malignancy.
 |
| FIGURE 20-2 Maxillary fibrosarcoma (FSA) is locally aggressive and can create considerable facial deformity. Golden retrievers in particular may have histologically low-grade, yet biologically aggressive FSA. |
BOX 20-3
CLINICAL SIGNS ASSOCIATED WITH ORAL TUMORS
• Ptyalism
• Halitosis
• Dysphagia
• Weight loss and/or decreased oral intake
• Mandibular or retropharyngeal lymph node enlargement
• Disinterest in chew toys
• Blood-stained food, water bowls, and bedding
• Exophthalmos
• Facial asymmetry
• Sneezing
• Nasal discharge
• Pawing at mouth
Diagnosis and Staging
Diagnostic methods and staging procedures depend upon tumor location and ease of sampling. Friable and rostrally located masses may be easily sampled under light sedation, whereas general anesthesia is suggested for biopsy of more caudal masses or those that are likely to bleed excessively. Before anesthesia, full physical examination, complete blood count, serum chemistries, and urinalysis are warranted. If malignancy is suspected, three-view thoracic radiographs are advised before other testing because detection of pulmonary metastatic disease warrants a poor prognosis and may influence case management decisions. Although the overall rate of pulmonary metastasis at the time of diagnosis is relatively low (14% or less for all oral malignancies), this finding means that local surgery is unlikely to be curative, and may therefore contraindicate aggressive surgery. 19 Radiographic or CT imaging of the affected site aids in determining tumor extent and in planning therapy. Over 50% of oral tumors arising from or near the gingiva have bone lysis demonstrable on radiographs. 19 Visual assessment may greatly underestimate the extent of the cancer, particularly feline oral SCC. In some cases, imaging may reveal the diagnosis, as was the case for the dog in Figure 20-3 . However, in most cases a biopsy is essential because inflammatory lesions and benign masses can look outwardly similar to malignant tumors. When lesions are small and superficial, excisional biopsy with curative intent is encouraged. Incisional biopsy is recommended for larger or invasive lesions, since treatment planning will vary depending on tumor histology and stage. Biopsy samples should be large and representative of the mass, with care taken not to sample too superficially or in necrotic areas. The biopsy site should be planned so that it can be excised at the time of definitive surgery. Lesions should be sampled via an oral approach, such that skin that may be needed later for wound closure using tissue flaps is not contaminated with tumor cells. 20 Use electrocautery only after biopsy specimens are obtained because cautery distorts the histologic appearance of the tissue. 15 A veterinary pathologist with immunohistochemistry (IHC) expertise should evaluate biopsy specimens. Useful IHC stains for poorly differentiated oral tumors are listed in Table 20-1 . 21,22 Melanomas may be difficult to differentiate from other malignancies because their cells can be round (like lymphoma and plasmacytoma), ovoid (like carcinomas), or spindle shaped (like sarcomas). Melanomas may also be amelanotic, thus lacking the granules that aid in diagnosis. For this reason, it is advisable to make impression smears of biopsy tissues before placing them in formalin so that cytologic evaluation can be compared with the histologic appearance.
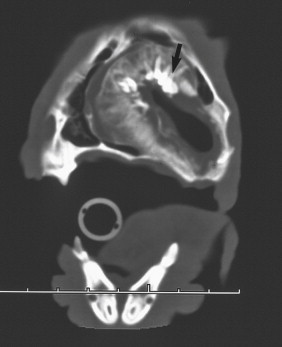 |
| FIGURE 20-3 Computed tomography scan of a dog with an odontoma reveals teeth (arrow) within the mass. In this case, imaging provided a diagnosis and surgery was curative. |
| FSA, Fibrosarcoma; SCC, squamous cell carcinoma. | |||
| IHC Stain | TUMOR TYPE | ||
|---|---|---|---|
| SCC | Melanoma | FSA | |
| Vimentin | – | +/– | + |
| Cytokeratin | + | – | – |
| S-100 | – | + | – |
| Melan A | – | + | – |
| HMB-45 and MEL-1 | – | + | – |
| Neuron-specific enolase | – | + | – |
Tumor staging should include fine-needle aspiration (FNA) of the regional lymph nodes (particularly those ipsilateral to the mass) even if they are not enlarged. The procedure should be performed after CT imaging, since FNA immediately before CT will cause artifactual changes in the appearance of the node on CT. Physical examination alone is a poor predictor of lymph node metastasis. 23,24 In one report, 40% of dogs with MM had nodal metastasis despite having normal-sized lymph nodes. 24 When nodal metastasis is suspected, based on imaging results or node enlargement, excision should be considered. There is no evidence that node excision facilitates distant metastasis. Furthermore, nodal biopsy may reveal metastasis that is not evident on cytology of an FNA sample. 25 It is important to remember that as well as mandibular nodes, the parotid and medial retropharyngeal nodes, although rarely palpable, also receive lymphatic drainage from the oral cavity. Imaging of all these regional nodes, in addition to the primary tumor, is warranted. 20,26
Metastasis
Of the oral malignancies encountered in dogs, MM is the most likely to metastasize, with a reported metastatic rate exceeding 80% ( Figure 20-4 ). 19 Feline oral MM is diagnosed infrequently, but has also been associated with pulmonary and other systemic sites of metastasis. 27 Regional lymph node metastasis is relatively uncommon with SCC, although canine tonsillar ( Figure 20-5 ) and canine lingual SCC are exceptions. 4,6,28,29 In some dogs with tonsillar SCC, regional lymph node enlargement caused by metastasis may be the reason for initial presentation, with the primary tumor only clinically evident later in the disease course. 6,30,31 The overall metastatic rate for canine FSA is less than 25%, with the regional lymph nodes or lungs most often affected. 5,18,19,32-34 Osteosarcoma (OSA) within the oral cavity is less likely to metastasize than appendicular OSA. 5,35,36 Benign oral lesions including epulides, ameloblastomas, and odontomas do not metastasize. However, left untreated, they will become quite large and impede normal function ( Figure 20-6 ).
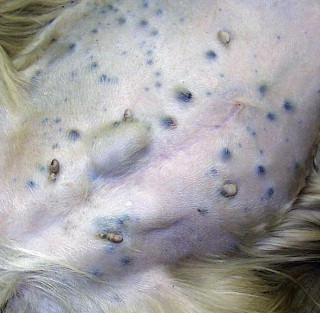 |
| FIGURE 20-4 Cutaneous metastasis on the ventral abdomen of a dog with oral malignant melanoma. |
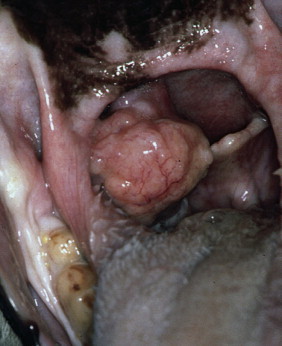 |
| FIGURE 20-5 Tonsillar squamous cell carcinoma as shown here warrants a poor prognosis due to its high metastatic rate and tendency to be detected late in the course of disease. |
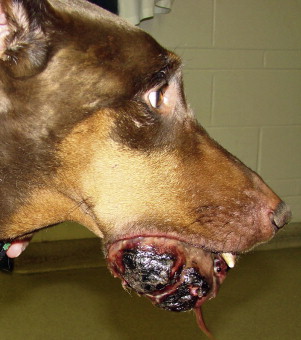 |
| FIGURE 20-6 Epulides, while not malignant, may be life-threatening due to expansive growth that can affect food intake. |
Treatment Modalities
Surgery
Complete surgical excision affords the best chance of a cure for most oral malignancies. Epulides do not metastasize and, if small, can be cured with excision alone. When multiple concurrent epulides occur in cats, they are more likely to recur after excision than in dogs; thus, wide excision is advised. 37 Rostrally located malignancies have the best prognosis; thus tumors in this location, including those on the tongue, should be excised with curative intent. 18 Even with excision of over half of the mobile portion of the tongue, dogs can maintain the ability to eat and drink. 6,28,38 Cats, on the other hand, may not tolerate extensive oral surgery as well and may have problems with prehension or compromised grooming ability after partial glossectomy. Results of surgery are summarized in Table 20-2 . 5,5,39-47 As long as surgical margins exceed 2 cm, complete maxillectomy or mandibulectomy may not be necessary. In one study, case outcome was similar regardless of the extent of surgery (radical vs. limited) for dogs with MM. 46 Considerable shrinkage of the surgical margins occurs after excision, formalin fixation, and slide preparation of lesions from the tongue and labiobuccal tissue. In fact, surgical margins of 8 to 10 mm are necessary in order to obtain clean histologic margins of 5 mm. 48 This should be taken into account when evaluating histopathology reports after resection and may alter interpretation of literature citing necessary surgical margins at these sites. Owner satisfaction with partial maxillectomy or mandibulectomy in dogs was 85% in one report. Owners perceived that the surgery afforded pain relief and were most pleased with results of rostral mandibulectomy, as compared with partial maxillectomy. 49 Gastrostomy tube placement may be an important aspect of postoperative and palliative care. Dietary management with gastrostomy tube placement is reviewed in Chapter 18, Section B . Cryosurgery can provide good results for small (<2 cm diameter) lesions with minimal bone invasion. 5,6,50,51 Complications such as oronasal fistula or bone necrosis and fracture may occur when cryosurgery is attempted for larger tumors.
| CR , Complete response; DFI , disease-free interval; MST , median survival time; PR , partial response; SCC, squamous cell carcinoma. | ||||
| Tumor Type | Outcome Measured | Surgery | Radiation Therapy | Chemotherapy |
|---|---|---|---|---|
| SCC | 1 year survival | Dogs: 70%–91% with mandibulectomy 25% for lingual | Cats: 30% with adjuvant mitoxantrone 57% with mandibulectomy | |
| Cats: <10% | ||||
| MST | Dogs: 18 mos | Dogs: 15–16 mos 34 mos when combined with surgery | Dogs: MST not reached with median follow-up of 534 days after piroxicam and carboplatin | |
| Cats: <6 mos with mandibulectomy 14 mos if surgery combined with radiation and gastrostomy tube placement | Cats: 60 days with hypofractionation 6 mos with adjuvant mitoxantrone 111.5 days with palliative fractionation and adjuvant gemcitabine | |||
| Response rate | Dogs: 17% with piroxicam 57% with carboplatin and piroxicam | |||
| Cats: 44% (1 CR and 3 PR in 9 cats) with doxorubicin and cyclophosphamide | ||||
| FSA | 1-year survival | Dogs: 23%–50% with mandibulectomy | ||
| MST | Dogs: 11 months | Dogs: 331 days with fine fractionation (52.5 Gy) 310 days with coarse fractionation (3 × 8 Gy or 5 × 6 Gy18–26 mos as adjuvant to surgery | ||
| MM | 1-year survival | Dogs: 21%–35% with partial mandibulectomy 27% with maxillectomy | ||
| MST | Dogs: 8–9.9 mos overall 5–10 mos with maxillectomy 7–17 mos with mandibulectomy | Dogs: 7 months 363-days with hypofractionation and cisplatin or carboplatin chemotherapy | Dogs: Carboplatin and surgery gave 57-day median DFI and 299-day mean DFI | |
| 511 days for lesions <2 cm and 164 days for lesions >2 cm222 days with lingual | Cats: 146 days with hypofractionation | |||
| Response rate | Dogs: 28% to carboplatin | |||
| Epulis | 1-year survival | Dogs: 90%–97% | Dogs: 75% with intralesional bleomycin | |
| MST | Dogs: 36–49 months | |||
Radiation Therapy
Radiation therapy may be used with curative intent for small SCC and epulides, for localized tumors with microscopically incomplete surgical margins, and as a palliative option for unresectable masses, including those crossing the midline of the hard palate. Results with radiation are summarized in Table 20-2 . 52-61 Response rates are generally best for SCC, although in one prospective clinical trial of megavoltage irradiation for 105 dogs with oral SCC, FSA, or MM, the only prognostic factor identified was tumor size regardless of tumor type. 52 Therefore, radiation therapy is most likely to be effective when used early in the course of treatment of oral tumors rather than for salvage therapy against large tumors late in their clinical progression. Although radiation response may be short-lived, it is sometimes possible to re-irradiate tumors that do respond, in hopes of achieving a second positive response. 53 Both coarse and fine fractionated radiation protocols (see Chapter 15, Section A for definitions) have been evaluated for treatment of oral tumors. Once-weekly fractionation provides similar clinical responses to standard fractionation for dogs with MM and those with non-resectable soft tissue sarcomas. 54,55 Accordingly, this option may be considered in an effort to prevent extended hospitalization. Coarse fractionation is not recommended for cats with oral cancer because of poor efficacy and unacceptable treatment complications. 56 Hyperthermia improves response to radiation therapy, but is labor-intensive and not readily available. 33
Medical Therapy
Because local disease progression, rather than metastasis, is often the reason for deaths associated with oral malignancies such as SCC and FSA, systemic chemotherapy may be less important than localized therapy. Adjuvant chemotherapy with mitoxantrone, 5-FU, and cisplatin has improved responses of human head and neck cancers to radiation. These drugs may also prove useful as adjuvant therapy for small animal patients, but published veterinary reports are rare. Neither cisplatin nor 5-FU should be used in cats because of the potential for drug-related fatal toxicity. Improved response rates for non-resectable feline oral SCC may be achieved by combining radiation and mitoxantrone (median survival time = 6 months; 30% 1-year survival ∗ ) or gemcitabine chemotherapy. 60 Chemotherapy alone has traditionally offered little benefit in terms of clinical outcome for oral tumors in dogs and cats. Carboplatin has been used to treat oral MM and SCC in dogs with non-resectable tumors. 62,63 The non-steroidal anti-inflammatory drug (NSAID), piroxicam, has shown some promise as a palliative treatment for oral SCC, both as single-agent therapy and in combination with chemotherapy. 63,64 A dose of 0.3 mg/kg/day PO in dogs and q48h in cats may be used if renal function is adequate. Because gastric ulceration may result from the use of NSAIDs, some advocate concurrent administration of a prostaglandin analogue such as misoprostol. Reported results with chemotherapy for oral malignancy are summarized in Table 20-2 . 5,8,59,60,62-70 In an unpublished report, a protocol combining bleomycin (20 mg/m 2 SC on days 1–3, then weekly for a maximum of 20 doses) and methotrexate (15–20 mg/m 2 SC weekly on a 4 weeks on/4 weeks off cycle) resulted in an 87.5% overall response rate in 8 cats with SCC (5 CR and 2 PR). ∗ Given this dramatic response rate and the reported lack of serious side effects, further evaluation of this protocol is warranted. Intralesional chemotherapy, usually with cisplatin, has been evaluated for treatment of oral tumors, including canine MM. 71,72 In one report, bleomycin (5 mg) was injected intralesionally once weekly to four dogs with recurrent acanthomatous epulis. All dogs responded, with complete responses in three dogs lasting over 1 year. 72 Intralesional chemotherapy is discussed in Chapter 12, Section E .
Photodynamic Therapy
Photodynamic therapy (PDT) (see Chapter 17 and Figure 14-2 ) shows promise in the treatment of oral SCC in dogs. 73,74 The treatment requires less hospitalization than radiation therapy and provides excellent cosmesis. In contrast, our experience with PDT for treatment of oral SCC in cats has been disappointing to date.
Immunotherapy/Gene Therapy
Immune enhancement techniques are the focus of active research and are covered in greater detail in Chapter 16 . For the oral tumors that occur in veterinary patients, immunotherapy is most promising for MM. 75-78 In 2007, the U.S. Department of Agriculture granted conditional licensure for a canine melanoma vaccine that is intended for use in the minimal disease setting, after the primary tumor has been locally controlled via surgery or radiation therapy. The vaccine is initially being made available only to board-certified oncologists.
Prognosis
Prognosis for dogs and cats with oral tumors is highly dependent upon tumor type and location. Table 20-2 summarizes outcome for various tumor types and treatment modalities reported in the veterinary literature.
SALIVARY GLAND TUMORS
Incidence—Morbidity and Mortality
Tumors of the salivary gland are uncommon in both the dog and cat with a reported incidence of 0.09% in the dog and 0.6% in the cat. 79 In a study of 245 salivary gland biopsy specimens, 42% of feline samples were neoplastic, whereas only 25% were neoplastic in dogs. 80 Benign tumors of the salivary gland are rare in both species. 79-82 A male sex predisposition may exist in cats 81 ; however, none has been shown in dogs. Siamese cats 81 and spaniel dogs 83 may be predisposed; however, this has not been substantiated in all reports. Salivary tumors appear to occur in older animals with a median age of 10 years in dogs and 12 years in cats. 81
Etiology and Risk Factors
Most salivary gland tumors in both dogs and cats are epithelial in origin with adenocarcinoma most common. 79-82,84 Other reported histologies include carcinoma, 79,80 acinic cell carcinoma, 81,82 SCC, 79,81,83 mucoepidermoid carcinoma, 82,83 cystadenocarcinoma, 3 mixed carcinoma, 79,82 basal cell adenocarcinoma, 85 sebaceous carcinoma, 86 salivary duct carcinoma, 87 pleomorphic adenoma, 79,80,88 FSA, 79,80 mast cell tumor, 79 lymphoma, 80,89 extraskeletal osteosarcoma, 90 and malignant fibrous histiocytoma (giant cell type). 91 No risk factors have been identified for the development of salivary gland tumors in dogs and cats.
Clinical Features
Most animals are presented for a non-painful swelling in the region of a salivary gland. Tumors can develop in either the major (mandibular, parotid, zygomatic, or sublingual) or minor salivary tissues throughout the oral cavity. The mandibular and parotid glands are most commonly affected in the dog and cat. 79-81,84 Bilateral salivary adenocarcinoma has been reported in a cat. 92 Other non-specific presenting complaints depending upon the salivary gland affected include halitosis, dysphagia, anorexia, weight loss, exophthalmos, the presence of Horner’s syndrome, dysphonia, and sneezing. 92,93
Diagnosis and Staging
FNA cytology is helpful in determining a diagnosis of salivary gland neoplasia. 92,94 Differential diagnoses to consider for salivary masses are included in Box 20-4 . 80,93 Because of the potential aggressive biological behavior for salivary tumors, a thorough physical examination, regional lymph node aspiration cytology, and thoracic radiographs are warranted at initial presentation. Skull radiographs may show a soft tissue swelling with or without periosteal reaction of surrounding bone. 93 Computed tomography (CT) is useful to help determine the invasiveness of the neoplasm and to assist the surgeon in planning resection. The World Health Organization TNM staging scheme is shown in Box 20-5 . 95
BOX 20-4
LIST OF POTENTIAL DIFFERENTIAL DIAGNOSES FOR SALIVARY GLAND ENLARGEMENT 80,93
Mucocele
Abscess
Salivary gland infarction
Sialadenitis
Sialolithiasis
Edema
Ductal ectasia
Primary neoplasia, malignant or benign
Metastatic neoplasia
BOX 20-5
WORLD HEALTH ORGANIZATION TNM STAGING FOR SALIVARY GLAND TUMORS 95
| T 1 | < 2 cm largest dimension |
| T 2 | 2-4 cm largest dimension |
| T 3 | > 4 cm largest dimension |
| N 0 | No nodal involvement |
| N 1 | Nodal involvement |
| M 0 | No distant metastasis |
| M 1 | Distant metastasis |
| Overall Tumor Stage | |
| I | T 1 N 0 M 0 |
| II | T 2 or T 3 , N 0 M 0 |
| III | Any T, N 1 M 0 |
| IV | Any T, any N, M 1 |
Metastasis
Metastasis may develop with salivary tumors in both dogs and cats. Cats are more likely to have advanced disease at the time of diagnosis compared with dogs. Hammer et al. showed that at the time of diagnosis; 39% of cats and 17% of dogs had nodal metastasis, and 16% of cats and 8% of dogs had visceral metastasis. 81 The lung is the most frequently reported distant metastatic site. 79,83.92
Treatment Modalities
When feasible, aggressive surgical resection is warranted. Unfortunately, many salivary neoplasms have extracapsular invasion and are locally extensive, making complete surgical excision unlikely. 82,93 Recurrence is common following surgical resection alone. Adjuvant radiation therapy has been reported and resulted in prolonged survival for three dogs with parotid salivary gland adenocarcinoma. 84 Because of the high potential for metastatic disease, chemotherapy is also warranted, particularly in cats. Drugs to consider should be based upon histologic diagnosis; either as single agents or alternating, doxorubicin and carboplatin are most often considered for salivary adenocarcinoma. Studies are lacking evaluating radiation and chemotherapy for the treatment of salivary neoplasms in companion animals; however, it is the authors’ opinion that the best chance for long-term control of a salivary gland tumor is aggressive surgical excision followed by radiation therapy and possibly chemotherapy.
Prognosis and Survival
Incompletely excised salivary tumors consistently recur and adjuvant radiation therapy can be helpful in preventing recurrence. 81,84 Reported median survival times (MSTs) for 24 dogs and 30 cats with salivary gland neoplasms was 550 days and 516 days, respectively. Dogs with stage I or II disease had significantly longer survivals than dogs with stage III or IV disease. Stage of disease was not, however, prognostic in cats. 81
Selected References ∗
J. Berg, Principles of oncologic orofacial surgery , Clin Techn Sm Anim Pract 13 ( 1998 ) 38 ;
This manuscript offers a thorough review of important considerations for orofacial surgery .
C.A. Carberry, J.A. Flanders, H.J. Harvey, et al. , Salivary gland tumors in dogs and cats: a literature and case review , J Am Anim Hosp Assoc 24 ( 1988 ) 561 ;
This is a comprehensive review of salivary gland tumors in dogs and cats .
J.P. de Vos, A.G.D. Burm, A.P. Focker, et al. , Piroxicam and carboplatin as a combination treatment of canine oral non-tonsillar squamous cell carcinoma: a pilot study and a literature review of a canine model of human head and neck squamous cell carcinoma , J Vet Comp Oncol 3 ( 2005 ) 16 ;
This pilot study provides the most encouraging results for chemotherapy of canine oral SCC to date .
R.S. Dhaliwal, B.E. Kitchell, S.M. Marretta, Oral tumors in dogs and cats. Part II. Prognosis and treatment , Compendium 20 ( 1998 ) 1109 ;
This review paper provides useful recommendations for treatment of canine and feline oral tumors .
S.M. Evans, D.E. Thrall, Postoperative orthovoltage radiation therapy of parotid salivary gland adenocarcinoma in three dogs , J Am Vet Med Assoc 182 ( 1983 ) 993 ;
This manuscript reports on response to radiation therapy of three dogs with parotid salivary gland adenocarcinoma .
A. Hammer, D. Getzy, G. Ogilvie, et al. , Salivary gland neoplasia in the dog and cat: survival times and prognostic factors , J Am Anim Hosp Assoc 37 ( 2001 ) 478 ;
This is the largest and most recent clinical study of salivary gland tumors in dogs and cats and evaluates location, survival time, and prognostic factors .
D.R. Proulx, D.M. Ruslander, R.K. Dodge, et al. , A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation , Vet Radiol Ultrasound 44 ( 2003 ) 352 ;
This large retrospective study showed no difference in response rate of MM to radiation based on fractionation scheme and established rostral tumor location, lack of bone lysis, and smaller tumor volume as predictors of a better prognosis .
J.A. Syrcle, J.J. Bonczynski, S. Monette, et al. , Retrospective evaluation of lingual tumors in 42 dogs: 1999-2005 , J Am Anim Hosp Assoc 44 ( 2008 ) 308 ;
This retrospective review highlights findings in dogs with tongue tumors and supports the previous report by Beck et al. 4 that lingual melanoma is more common than lingual SCC in dogs .
SECTION B Nasal Tumors
Mary Lynn Higginbotham and Carolyn J. Henry
KEY POINTS
• Dogs with intranasal tumors are often presented for unilateral or bilateral nasal discharge that may be hemorrhagic and is often initially antibiotic responsive.
• Nasal discharge is less common in cats with intranasal tumors than dogs; epiphora and facial deformity are often associated with feline intranasal tumors.
• Radiation therapy is the primary treatment for canine intranasal tumors, with a median survival time of approximately 1 year.
• Lymphoma is a common intranasal feline tumor that has a good response to multi-modal therapy and median survival time in excess of 2 years.
• Nasal planum squamous cell carcinomas are locally invasive tumors of dogs and cats that carry a good long-term prognosis with early intervention and aggressive local therapy.
Incidence—Morbidity and Mortality
Nasal tumors may be intranasal —arising from the tissues inside the nasal cavity such as the epithelial lining, cartilage, bone, or lymphoid tissues— or they may be extranasal —arising from the nasal planum or other tissues covering the nose. The majority of canine and feline nasal tumors are malignant. 1 Common intranasal tumors include various carcinomas and sarcomas as well as lymphoma ( Box 20-6 ). Adenocarcinoma is the most common intranasal tumor in the dog 1-3 whereas lymphoma is most common in the cat. 1,4,5 SCC is the most frequently encountered tumor of the nasal planum in both species.
BOX 20-6
MOST FREQUENTLY DIAGNOSED NASAL TUMORS IN DOGS AND CATS
Adenocarcinoma
Squamous cell carcinoma
Non-keratinizing squamous cell carcinoma (transitional carcinoma)
Undifferentiated carcinoma
Neuroendocrine carcinoma
Chondrosarcoma
Osteosarcoma
Fibrosarcoma
Hemangiosarcoma
Mast cell tumor
Lymphoma
Transmissible venereal tumor
Etiology and Risk Factors
Long-nosed dogs (dolichocephalic breeds) are suspected to be at increased risk for intranasal tumor development. 1 Exposure to environmental tobacco smoke, use of topical insecticides, and exposure to indoor coal or kerosene heaters have been associated with an increased risk of developing intranasal cancer. 6,7 Cyclooxygenase-2 (COX-2) expression has been found to be upregulated in canine intranasal carcinomas and may play a role in the pathogenesis of this disease. 8-10 Exposure to ultraviolet (UV) light is a risk factor for the development of nasal planum tumors in cats. 11
Clinical Features of Canine Intranasal Tumors
Clinical signs associated with intranasal tumors are due to erosion of the mucosa, destruction of the turbinates, and invasion into the surrounding structures by the tumor ( Box 20-7 ). The majority of animals with intranasal tumors are presented with nasal discharge that initially responds to antibiotics. Clinical signs often recur soon after the discontinuation of the antibiotic. Adenocarcinoma is the most frequently diagnosed intranasal tumor in the dog. 1-3 Metastasis at the time of diagnosis is rare, 12 but approaches 40% in dogs that succumb to the disease. 13 Lymph nodes and lungs are the most common metastatic sites, but other sites including skin 14 and bone 14,15 have been documented. Paraneoplastic hypercalcemia has been reported in dogs with nasal carcinoma. 16,17
BOX 20-7
CLINICAL SIGNS ASSOCIATED WITH INTRANASAL TUMORS
Unilateral or bilateral epistaxis
Unilateral or bilateral mucoid to mucopurulent nasal discharge
Sneezing
Reverse sneezing
Epiphora
Facial deformity
Neurologic signs
Chondrosarcoma (CSA) is the most common intranasal mesenchymal tumor in dogs. 1 Metastasis at the time of diagnosis of nasal sarcomas is uncommon. Paraneoplastic erythrocytosis has been documented in one dog with a nasal FSA. 18
Clinical Features of Feline Intranasal Tumors
Lymphoma is the most frequently diagnosed feline intranasal tumor and often occurs in older cats. 5 It can cause significant erosion of the nasal or frontal sinus cavities, resulting in severe facial deformity. The majority of cats with nasal lymphoma do not have evidence of systemic disease at the time of diagnosis; however, up to 20% of cats will have disease progression outside of the nasal cavity with time. 19,20 Intranasal carcinomas also occur in cats. Metastasis at diagnosis is uncommon.
Clinical Features of Canine Extranasal Tumors
Extranasal SCC is most often associated with the nasal planum in dogs. Metastasis at the time of diagnosis is rare. 21,22
When occurring within the extranasal tissue, lymphoma typically appears as a non-pigmented plaque associated with the mucocutaneous junction and nasal philtrum. Lymphoma in this site is often epitheliotrophic in nature, is of T-cell origin, and may be the only detectable disease at the time of diagnosis. 23 In general, however, lymphoma is considered a systemic disease, and thorough staging is recommended.
Clinical Features of Feline Extranasal Tumors
SCC is the most common feline extranasal tumor. Cats with nasal planum tumors are usually light skinned, white-haired cats that may also have lesions associated with their pinnae and conjunctiva resulting from chronic UV light exposure. These solar-induced lesions progress from what is described as actinic keratosis to non-invasive carcinoma in situ (where the tumor does not invade the epidermal basement membrane) to invasive SCCs. 11 These tumors are often confused with non-healing wounds early in the course of disease when they are ulcerated, scabbed lesions. Metastasis is uncommon and treatment is aimed at controlling local disease. 24
Diagnosis and Staging
Physical Examination
For animals presenting with clinical signs associated with the upper airway, close attention should be paid to assessment of air flow through the nostrils, ocular examination, oral examination, and regional lymph node examination. Air flow may be assessed using either a glass slide or cotton ball and evaluating for condensation on the slide or movement of the cotton as air flows from the nasal passage. The patient should be assessed for facial deformity ( Figure 20-7 ) that indicates erosion of the nasal bone and infiltration into the extranasal soft tissues. Decreased retropulsion of either or both of the eyes suggests that the mass has eroded into the orbit. Ocular discharge may also be a sign of orbital compromise because of an invasive nasal tumor. An oral examination should be performed to assess for asymmetry of the hard and soft palates. Careful lymph node palpation should be performed, paying special attention to mandibular and prescapular nodes. Lymph node aspiration and cytology are recommended even if nodes are not considered palpably abnormal.
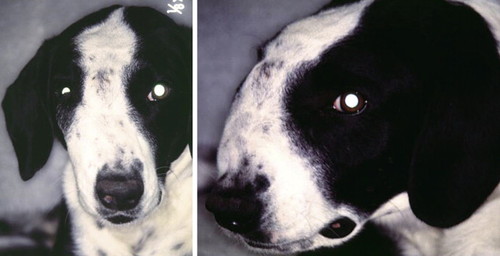 |
| FIGURE 20-7 Facial deformity may be a feature of a nasal malignancy that has eroded through the nasal bone and infiltrated the extranasal soft tissues. (Photos courtesy of Dr. Eric Pope.) |
Bloodwork
A minimum database including a CBC, biochemical profile, and urinalysis should be performed in all patients suspected of having a nasal tumor. A coagulation profile and blood pressure measurement should be done for animals presenting with epistaxis in order to rule out etiologies other than primary nasal cancer.
Imaging Studies
Thoracic radiographs should be evaluated for the presence of pulmonary metastatic disease. Skull radiographs may be helpful in evaluating for a nasal mass, although lack of radiographic evidence of a nasal tumor does not rule out its presence. CT is superior to radiography when evaluating the nasal cavity and is recommended over radiographs when one has a high index of suspicion for a nasal malignancy. 25 FIGURE 20-8 and FIGURE 20-9 offer comparison of radiography and CT for identification of a nasal mass in a dog. In addition to confirming the presence of a mass, a CT scan will enable assessment of the local disease and provide the images necessary for radiation treatment planning. Disease stage is prognostic for dogs who are treated with radiation therapy for intranasal tumors. 26,27 It is, however, important to remember that the CT scan should be performed before biopsy and aspiration of regional lymph nodes in order to prevent iatrogenic contrast enhancement that could be misinterpreted as neoplastic disease on the CT images.
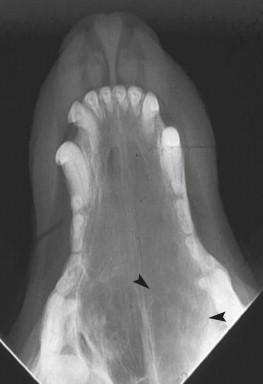 |
| FIGURE 20-8 Intraoral radiograph of the nasal cavity in a dog with an intranasal adenocarcinoma. Note the increased soft tissue opacity in the caudal aspect of the right side of the nasal cavity. No radiographic evidence of lysis of the nasal septum or other structures is evident in this film. (Photo courtesy of the University of Missouri Veterinary Oncology Service.) |
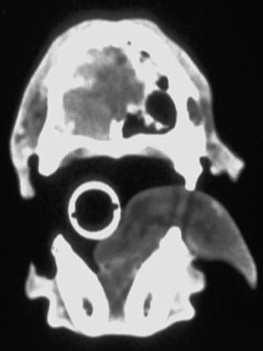 |
| FIGURE 20-9 CT image of the dog in Figure 20-8 . This nasal mass appears to originate in and consume the right nasal cavity. Note the erosion of the nasal septum and the extension of the mass into the left nasal cavity. (Photo courtesy of the University of Missouri Veterinary Oncology Service.) |
Rhinoscopy
Rhinoscopy is useful for visualizing a mass before a transnasal biopsy, as well as for assessing for fungal plaques, nasal mites, or other non-neoplastic causes of nasal disease. As with FNAs and biopsies, rhinoscopy may cause hemorrhage that leads to subsequent contrast enhancement on CT images. As such, rhinoscopy should follow, rather than precede, CT when both imaging modalities are used.
Biopsy and Histopathology
Histopathology is necessary to confirm a diagnosis and may be facilitated by transnasal biopsy. This technique is preferred over an approach through the skin, since it minimizes the possibility for creating a biopsy tract that will necessitate skin excision at a later date. Alligator forceps or forceps with a round cup biopsy tip are passed through the nares to the level of the mass, as indicated on CT scan or until resistance is met. The biopsy should be taken at this location. Before beginning the procedure, it is recommended to mark the biopsy instrument with tape or a marker at the depth of the medial canthus of the eye by aligning the instrument with the bridge of the nose to measure the distance from the nares to the caudal aspect of the nasal cavity ( Figure 20-10 ). This will prevent inadvertently passing the biopsy instrument through the cribriform plate. A minimum of five tissue samples should be obtained in order to provide an adequate and representative sample for the pathologist to review. Aspiration 3 and hydropulsion ∗ techniques have also been described to obtain nasal biopsies. The hydropulsion technique was successful in obtaining tissue in 92% of cases and provided clinical relief to the patient by non-invasive tumor debulking. ∗
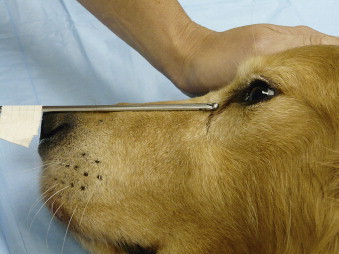 |
| FIGURE 20-10 Before obtaining a transnasal biopsy sample, it is important to mark the biopsy instrument to indicate the length to the medial canthus. This will prevent inadvertent advancement of the biopsy instrument through the cribriform plate. (Photo courtesy of University of Missouri Veterinary Oncology Service.) < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|