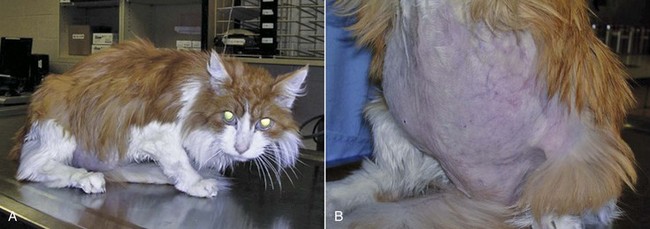
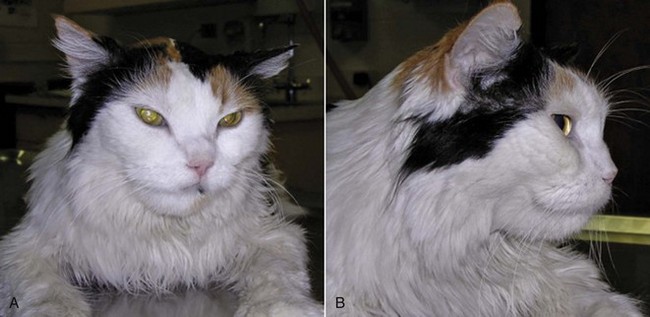
25 Endocrine tumors may be classified as nonneoplastic hyperplasias, benign adenomas, or malignant carcinomas. These conditions represent points on a continuous spectrum of endocrine function-dysfunction. Nonneoplastic hyperplasia is the consequence of aberrant secretion of trophic hormones resulting in growth and increased function. Hyperplasia is often focal but not necessarily reversible when the inciting trophic factor is removed, illustrating the overlap between nonneoplastic and neoplastic endocrine disease.1 Adenomas and carcinomas grow autonomously in the absence of trophic stimulation. Distinguishing between benign and malignant lesions can be challenging. In particular, the cellular morphologic features of adenomas and carcinomas are often similar, making cytologic samples, small biopsies, and even entire tumors difficult to accurately classify.1 The clinical manifestations of endocrine tumors may be the result of growth, expansion, and metastasis of the tumor, producing traditional sequelae due to compression of normal tissue (adenoma), invasion and destruction of regional or systemic normal tissue function (carcinoma), secretion of hormones or hormone-like substances that produce signs of disease specific to the downstream tissue impact, and often a combination of these effects. In this chapter, we will review the common endocrine tumors of dogs and cats. A discussion of endocrine neoplasia in ferrets is beyond the scope of this chapter; however, a thorough review is available for the interested reader.2 Multiple endocrine neoplasia is not discussed here because it remains rarely reported in companion species. Endocrine tissue is distinct from nonendocrine tissue in several ways that influence the pathogenesis of cancer. Endocrine glands normally expand in secretory capacity by an increased number of cells, increase in size and productivity of individual cells, or both, when the necessary stimulus is applied. They then return to a relatively quiescent state when the trophic substance is removed. Growth (cellular enlargement and division) and function (production of hormone) are therefore tightly linked and controlled by the same physiologic stimulus. Cells within endocrine organs are relatively stable. For example, the thyroid gland is expected to have only six to eight renewals in the lifespan of an adult human, dog, or mouse.3 This low turnover rate (2.3 years in dogs) is associated with longer intermitotic intervals compared to many other cell types in nonendocrine tissues. Such quiescence may result in a lower mutation rate, although any mutations acquired may be retained for longer periods.4 Prolonged stimulation of mutation-bearing endocrine tissue by a trophic substance may result in transformation. Clinically, this may be observed when longstanding hyperplasia ultimately progresses to neoplasia. In this situation, growth and function within the endocrine organ ultimately become independent of trophic hormone stimulation. The orchestrated accumulation of somatic mutations leading to dedifferentiation from mature to anaplastic cells is considered the classic theory of carcinogenesis in thyroid tissue, and this area is where the majority of research has occurred.5 Loss of tumor suppressor gene function, or oncogene activation, may lead to neoplasia. Only recently has a cancer stem cell theory of carcinogenesis emerged for endocrine neoplasia, in particular for thyroid neoplasia.6–9 Although a cancer stem cell has not yet been isolated from human thyroid cancer tissue, the strong appearance of an epithelial-to-mesenchymal transition in the morphology of thyroid anaplastic carcinoma suggests the potential of cancer stem cells. Additional well-known pathways of carcinogenesis include the germline alterations predisposing to tumor development and conventional agents such as exposure to ionizing radiation or environmental exposures to endocrine disruptors and thyroid mimics. Growth factors such as growth hormone (GH), insulin-like growth factors (IGFs), and epidermal growth factor (EGF) also play an integral role in the pathogenesis of endocrine neoplasia.10 However, normally differentiated and neoplastic endocrine cells respond similarly to growth factor stimulation, and it is unlikely that any single growth factor can cause transformation of a cell. The role of growth factors within endocrine oncogenesis therefore is thought to be that of a tumor promoter, whereby growth factor–stimulated hyperplasia increases the probability of mutational events that may eventually release the cell from growth control. Nonfunctional pituitary tumors become clinically significant when they are large enough to cause neurologic signs, including obtundation, stupor, behavioral changes, decreased appetite, gait abnormalities, seizures, blindness, and other cranial nerve abnormalities.11–14 In one case series, pituitary tumors were the second most common type of secondary brain tumor, accounting for 25% of 177 cases.15 The pituitary gland may also be affected by secondary tumors, either through direct extension or by metastatic spread from a distant site.1 Locally invasive or compressive primary or secondary pituitary tumors also have the potential to cause loss of pituitary function, resulting in hypothyroidism, hypocortisolism, gonadal atrophy, or central diabetes insipidus.16 Hypercortisolism (HC), also termed hyperadrenocorticism or Cushing’s syndrome, is a common endocrine disease of middle-aged and older dogs.17 It is uncommon in cats. This clinical syndrome results from chronic exposure to excessive blood levels of glucocorticoids. Naturally occurring canine and feline HC are almost always either pituitary-dependent or a result of excessive glucocorticoid secretion from an adrenocortical tumor.17,18 HC in human medicine is differentiated into ACTH-dependent and ACTH-independent disease.19 ACTH-dependent disease most commonly results from excessive ACTH secretion from a pituitary tumor but may also be caused by ectopic secretion of ACTH from an extrapituitary tumor or very rarely by ectopic secretion of corticotrophin-releasing hormone (CRH). There is only one clearly documented report of ectopic ACTH secretion in the dog,20 which may reflect the fact that it is very difficult to prove this diagnosis. Pituitary-dependent hypercortisolism (PDH) is the most common form of spontaneous HC in dogs and cats, accounting for 80% to 85% of cases in these species.17,18,21,22 This disorder is a consequence of autonomous synthesis and secretion of ACTH from a pituitary tumor. The secretion of ACTH from the pituitary tumor is chronically excessive, leading to bilateral adrenal cortical hyperplasia and hypercortisolemia. The pituitary tumor is relatively insensitive to negative feedback by cortisol and there is also a loss of hypothalamic control over ACTH release because CRH secretion is suppressed by the chronic hypercortisolemia.18 Pituitary tumors that secrete ACTH are derived from pituitary corticotroph cells. Expression of several proteins and their receptors thought to be involved in the etiology of human pituitary tumors responsible for ACTH hypersecretion has been studied. Leukemia inhibitory factor and its receptor are expressed in canine pituitary adenoma samples, although no mutations were identified in the receptor that might account for autonomous ACTH production.23,24 Dopamine and somatostatin receptor subtype expressions were evaluated in normal canine pituitary tissue and compared to adenomatous tissue. The type 2 dopamine receptor and somatostatin type 2 (SST2) receptor were most prevalent in canine pituitary tumors but expressed at low levels. Pituitary tumors may be described as macrotumors or microtumors.18 The latter distinction is derived from human medicine: microtumors are less than 1 cm in diameter and macrotumors are 1 cm or larger in diameter. The use of this size-based classification is controversial in veterinary medicine, at least partly due to variability in patient size and conformation.12 Pituitary tumors may also be classified as noninvasive adenomas, invasive adenomas, or adenocarcinomas. The latter term is reserved for tumors in which there is demonstrated evidence of metastatic disease. Canine pituitary adenocarcinomas are uncommon. A recent study of 33 dogs with pituitary tumors, all of which underwent necropsy evaluation after brain imaging, revealed that 61% had a pituitary adenoma, 33% had an invasive adenoma, and 6% had an adenocarcinoma.11 The majority of dogs with PDH are older than 9 years of age, and female dogs are slightly overrepresented. Breed predispositions have been noted in Dachshunds, terrier breeds, German shepherd dogs, and poodle breeds.17,18 The onset of canine Cushing’s syndrome is often slow, and the signs may progress slowly. Affected dogs are often not considered by their owners to be sick; they have a good appetite and do not show signs such as vomiting, diarrhea, coughing, or weight loss. Because spontaneous HC typically affects elderly dogs, the signs may initially be attributed to normal aging. The progress of the disorder is generally insidious, but eventually the owners of affected dogs seek veterinary care due to the frustration associated with signs such as polyuria, polydipsia, panting, and exercise intolerance. The most common signs of canine HC are polyuria, polydipsia, polyphagia, abdominal enlargement, lethargy, panting, exercise intolerance, muscle weakness, alopecia, calcinosis cutis, thinning of the skin, and reproductive abnormalities.17,18 These clinical signs are the result of the gluconeogenic, catabolic, immunosuppressive, and antiinflammatory effects of excessive circulating glucocorticoids. Glucose intolerance and insulin resistance have been shown to be common in dogs with HC,25 and overt diabetes mellitus may develop in as many as 10% of these patients.26 The catabolic effects of glucocorticoids result in thinning of the skin, poor wound healing, muscle wasting, and decreased bone density.27 The antiinflammatory and immunosuppressive properties of glucocorticoids are responsible for the increased susceptibility to infections in dogs with hypercortisolemia.17 This most often manifests as an increased incidence of urinary tract infections in canine patients. In one study, 46% of dogs with HC were found to have a urinary tract infection.28 More serious disorders associated with canine HC include hypertension and proteinuria.17,29 These are particularly insidious because they may not initially be associated with overt clinical signs. If untreated, hypertension can damage end organs such as the eye, brain, and kidneys, leading to complications that may include blindness or glomerular disease. Over 80% of dogs with canine Cushing’s syndrome were reported to be hypertensive in one case series.30 Although uncommon, pulmonary thromboembolism is another potentially life-threatening complication of HC.26,31 Spontaneous HC is suspected when typical clinical signs are detected in a middle-aged or older dog that is not receiving exogenous glucocorticoid therapy. The diagnosis is further investigated when initial laboratory tests reveal classic abnormalities, including neutrophilia, monocytosis, lymphopenia, eosinopenia, thrombocytosis, elevated serum alkaline phosphatase levels, a mild increase in alanine aminotransferase, and elevated cholesterol. Urinalysis may demonstrate minimally concentrated, isosthenuric, or hyposthenuric urine. Ancillary tests recommended in dogs suspected to have HC include urine culture, blood pressure measurement, thoracic radiographs, and abdominal ultrasonography.17,18,32 The tests that are most commonly used to screen for the presence of spontaneous HC are the urine cortisol : creatinine ratio, the ACTH stimulation test, and the low-dose dexamethasone suppression test (LDDST). These tests should be reserved for patients in which there is clinical suspicion of HC. Due to space considerations, readers are directed to several excellent references for more in-depth discussion of the comparative aspects of these tests.17,18,32,33 The urine cortisol : creatinine ratio is very sensitive, but specificity is low and it should not be used in patients with concurrent illnesses. The ACTH stimulation test has a lower sensitivity and higher specificity in comparison to the LDDST. The LDDST is highly sensitive and therefore less likely to give false-negative results. An additional advantage of this test is that it is capable of distinguishing between PDH and ADH in some cases.17 For patients with typical clinical signs of HC and positive results on the ACTH stimulation test, further testing is generally performed to differentiate between pituitary and adrenal-dependent disease. These further tests are also necessary after positive results on the LDDST for those patients in which this test does not additionally confirm the presence of PDH. Differentiation tests that are commonly used include the high-dose dexamethasone suppression test (HDDST) and the measurement of endogenous ACTH levels.17,32 In dogs with ADH, it is expected that endogenous ACTH levels will be low due to feedback inhibition of pituitary ACTH release by chronic hypercortisolemia. Patients with PDH would be expected to have elevated endogenous ACTH levels.34–36 The results of imaging studies, including ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI), may assist in differentiating between PDH and ADH.17,18 Abdominal ultrasonography should not be used as a screening test for HC, and it should also not be used as the sole mechanism for discriminating between PDH and ADH; however, it can provide useful information.35,37–40 The adrenals of patients with PDH are often bilaterally enlarged with increased thickness, and they typically maintain a normal shape and are homogeneous in echogenicity.37 However, there can be overlap between adrenal gland measurements in normal dogs, dogs with nonadrenal disease, and dogs with HC. Adrenal gland asymmetry may also be detected in dogs with PDH due to nodular hyperplasia. In some cases, this appearance can be confused with adrenal neoplasia. To further complicate the diagnostic accuracy of abdominal ultrasonography, a small percentage of patients with HC may have concurrent PDH and ADH.41 Bilateral adrenal tumors may also occur, including both functional or nonfunctional adrenocortical tumors and pheochromocytomas.39,42–45 Thus ultrasound findings must always be interpreted concurrently with clinical findings and endocrine test results. Abdominal CT is used less commonly than ultrasonography to evaluate the adrenals, but CT findings may also assist in the discrimination between PDH and ADH.46,47 This technique also demonstrates overlap between adrenal volume in dogs with PDH and dogs with nonadrenal disease and also confirms that dogs with PDH can have nodular adrenal lesions.47 Although 80% to 85% of dogs with spontaneous HC have PDH and almost all cases of PDH are due to the presence of a pituitary tumor, canine patients do not often show clinical signs directly referable to the local effects of the tumor.17 The vast majority of cases are initially presented for veterinary care due to the typical clinical signs of HC, as described previously. Pituitary tumors may be detected by CT,12,14 dynamic CT,48,49 MRI,12,14,50–53 or dynamic MRI54; however, these techniques are not routinely performed in all dogs that are diagnosed with PDH.12 In most cases, the diagnosis is based on the presence of typical clinical signs and clinicopathologic changes of hypercortisolemia, together with the results of endocrine testing.17 As noted previously, brain imaging is not performed in the great majority of dogs with PDH, and most receive treatment to address adrenal hyperfunction rather than the pituitary tumor itself. This is most likely due to the fact that brain imaging and pituitary surgery or radiation therapy (RT) are not affordable or accessible to many clients. Although medical therapy for PDH (see later) has a long history of successful use, it is important to note that the pituitary lesion in dogs with PDH will progress over time. In a study of 13 dogs that underwent MRI evaluation of the brain at the time of diagnosis of PDH and before medical therapy was instituted, 8 of the dogs had a visible pituitary mass and none of the dogs had clinical signs of neurologic disease.53 Four of the dogs showed enlargement of the pituitary tumor on MRI 1 year later, and a pituitary tumor was also detected in two dogs that did not have a visible mass on the initial MRI. Two of the 13 dogs had developed neurologic signs at the time of the 1-year follow-up MRI. A recent study evaluated diagnostic imaging findings in 157 dogs with PDH with and without neurologic signs.14 Central nervous system (CNS)-specific signs such as circling, seizures, or ataxia were neither sensitive nor specific for predicting the presence of a pituitary macrotumor. However, signs such as lethargy, mental dullness, and decreased appetite were highly specific for detection of a pituitary macrotumor but not highly sensitive. Other studies have also documented that mentation and appetite changes are the most common signs associated with pituitary tumors.13,55 When considering brain imaging in dogs with PDH, several factors should be taken into account: 40% to 50% of dogs with PDH have tumors that are not visible on CT or MRI, and these dogs are unlikely to develop neurologic signs associated with the tumor; 15% to 25% of dogs with PDH are at risk for the development of neurologic signs due to the presence of an enlarging tumor, and these signs typically develop within 6 to 18 months of the diagnosis of PDH; brain imaging may be helpful in predicting dogs likely to develop neurologic signs in patients with PDH that initially have no signs directly attributable to the tumor17; and if RT is being considered, early treatment will likely improve prognosis.13,56 It is also possible that measurement of plasma ACTH precursor concentrations could help in the selection of patients for brain imaging because it has been shown that pro-opiomelanocortin/pro-ACTH levels in plasma are correlated with pituitary tumor size in dogs with PDH.57,58 However, plasma cortisol concentrations at baseline and 4 or 8 hours after administration of a low dose of dexamethasone do not appear to correlate with the development of neurologic signs.14 Hypophysectomy is the treatment of choice for PDH in humans and can be successful in dogs.59–61 A recent prospective study of 150 dogs that underwent transsphenoidal hypophysectomy for PDH gave 1-, 2-, 3-, and 4-year estimated survival rates of 84%, 76%, 72%, and 68%, respectively. Twelve dogs died postoperatively and 127 went into remission, with 32 of those dogs later experiencing a recurrence of disease. Complications included central diabetes insipidus in 53% of dogs undergoing remission and incomplete hypophysectomy in nine dogs. The overall success rate of transsphenoidal hypophysectomy was determined to be 65% in this study.61 Unfortunately, this approach requires a high degree of specialized surgical skill and experience, and it is not readily available outside of one center in Europe. There are several reports of the successful use of radiation in the treatment of canine PDH.13,56,62–64 Dow and colleagues treated six dogs with functional pituitary macrotumors with 40 Gy given in 10 equal fractions. Median survival was reported to be 743 days, neurologic signs resolved in all dogs, and ACTH levels remained high for at least 1 year after therapy.62 Goossens et al used cobalt 60 RT to treat six dogs with PDH caused by a pituitary tumor that was detectable on MRI, and tumor size was significantly reduced in all cases. However, clinical signs of PDH were only adequately controlled in one dog.63 Théon and Feldman evaluated the effects of megavoltage irradiation on pituitary tumors in 24 dogs with neurologic signs. Ten dogs experienced complete remission of neurologic signs, and another 10 dogs achieved partial remission. As in previous studies, these authors noted a correlation between relative tumor size and the severity of neurologic signs in dogs with pituitary tumors. They also noted a correlation between tumor size and remission of neurologic signs after pituitary irradiation, suggesting that early treatment of these tumors should improve prognosis, although control of ACTH secretion was unlikely.56 A recent retrospective study of RT for the treatment of pituitary masses demonstrated significantly improved survival times and control of neurologic signs in 19 dogs that received RT, compared to 27 untreated control dogs with pituitary masses. Mean survival time in the treated group was 1405 days, compared to 551 days in the nonirradiated group. The 1-, 2-, and 3-year estimated survival rates were 93%, 87%, and 55% for the irradiated and 45%, 32%, and 25% for the nonirradiated dogs, respectively. Treated dogs with smaller tumors lived longer than those with larger tumors, again suggesting that early diagnosis and treatment of pituitary tumors are beneficial. Five of 14 dogs with PDH in this study were reported to show resolution of clinical signs of HC together with at least one normal ACTH stimulation test result after completion of RT.13 Most of the reports that document the use of RT for the treatment of pituitary tumors in dogs provide little detailed information regarding the progress of the clinical syndrome of HC in these patients.13,56,62–64 Thus, although RT appears effective in controlling neurologic signs and increasing survival,13 it is difficult to predict the endocrinologic outcome of RT for dogs with PDH. A detailed review of medical therapy for PDH is beyond the scope of this chapter, and the information here should be regarded as a brief introduction. Readers are strongly encouraged to consult any of several excellent discussions of this subject before initiating medical therapy in any patient.17,18 The two medications that are most commonly used to treat PDH in dogs are mitotane and trilostane. Other drugs such as ketoconazole, l-deprenyl, metyrapone, bromocriptine, and retinoic acid are used rarely and will not be discussed further in this chapter. Mitotane (o,p′-DDD, Lysodren) is a potent adrenocorticolytic agent that is cytotoxic to the adrenal cortex, particularly the zona fasciculata and zona reticularis. Mitotane therapy is most commonly divided into a loading or induction phase that typically lasts between 5 and 9 days, followed by maintenance therapy. Patients should be monitored very closely during loading with mitotane, and this phase is regarded as being complete when the post-ACTH cortisol is less than 5 (or 4) µg/dL on an ACTH stimulation test. This usually coincides with a decrease in appetite or in polyuria/polydipsia in the patient, but not always. Thus an ACTH stimulation test should be performed as soon as the patient shows any change in clinical signs or within 5 to 7 days of starting mitotane, whichever occurs first. A typical induction dose of mitotane is 30 to 50 mg/kg day, and maintenance therapy with mitotane typically requires giving the initial induction dose weekly, although the exact dose depends on how the patient responded to the induction course. Thus a patient requiring 500 mg per day for induction will often be started on 500 mg weekly for maintenance, with the weekly dose typically divided between 2 to 4 days. The mitotane dose should always be divided when possible and given with food, and clients should be instructed to wear gloves when handling the medication. An ACTH stimulation test is typically performed 1 month after initiation of maintenance therapy and then several times a year thereafter. The frequency of testing will depend on the dog’s clinical signs and the frequency with which dose adjustments are made. An alternate mitotane protocol has also been described for dogs with PDH. In contrast to the selective adrenocorticolysis protocol summarized earlier, which is designed to spare the zona glomerulosa, mitotane can also be used to achieve nonselective complete adrenocorticolysis. In this protocol, the medication is given at a higher dose for a fixed time period, and glucocorticoid and mineralocorticoid supplementation are started shortly after initiation of mitotane therapy and continued thereafter.65 This approach may be preferred in dogs that relapse frequently on the selective protocol and in dogs with concurrent diabetes mellitus. It may also be less expensive in some patients. However, this protocol was reported to cause adverse effects requiring temporary cessation of therapy in 29% of dogs, and relapse occurred in 39% of dogs that underwent remission.65 Thus the use of this protocol does not eliminate the need for careful long-term follow-up. Trilostane (Vetoryl) is an orally active synthetic corticosteroid analog that competitively inhibits 3-β-hydroxysteroid dehydrogenase.66 This enzyme is essential for synthesis of cortisol and other steroids such as corticosterone, androstenedione, and aldosterone. Trilostane has been used in Europe for several years for the management of canine PDH and recently was approved for use in the United States. Initial publications suggested a starting dose of trilostane of around 6 mg/kg once daily, with the first follow-up ACTH stimulation test performed at 10 to 14 days.67 As more experience has been gained with this medication, it has become apparent that much lower starting doses may be effective and potentially associated with fewer adverse effects.68,69 It is also appears that trilostane should be used twice daily in most dogs,70 with a recent study suggesting a starting dose in the range of 0.2-1.1 mg/kg every 12 hours.68 The response to trilostane is also monitored with ACTH stimulation testing, with the aim of achieving a post-ACTH cortisol value in the range of 1.5 to 5 µg/dL. Values slightly above this range are acceptable if the patient’s clinical signs are well controlled.66 It is recommended that the ACTH stimulation test be started 3 to 4 hours after trilostane is given for dogs receiving twice daily therapy. Although most authors suggest performing the first ACTH stimulation test at 10 to 14 days, the reason for this is unclear because the results of this test generally continue to improve beyond 2 weeks, even when the dose is not changed. Both mitotane and trilostane are highly effective for the treatment of canine PDH. A retrospective comparison of mitotane and trilostane in dogs with PDH demonstrated a median survival time (MST) of 662 days for trilostane and 708 days for mitotane.71 It is important to note that both mitotane and trilostane can have adverse effects in dogs. Both medications can be associated with anorexia, vomiting, or diarrhea, and both can cause hypoadrenocorticism.17,18,66 Trilostane therapy has also been associated with adrenal necrosis.72,73 It should also be noted that the adrenal glands of dogs receiving mitotane therapy for PDH will become smaller over time, whereas the adrenal glands of dogs on trilostane therapy have been shown to increase in size by 6 weeks after initiation of therapy and may develop an irregular nodular ultrasonographic appearance after several months of therapy.74 In deciding whether to use trilostane or mitotane in a patient with PDH, the clinician should consider his or her own comfort level and experience with each medication and also the wishes of the client. A cost analysis should also be performed, including the cost of follow-up office visits and ACTH stimulation testing, as well as the cost of the medication itself. It is recommended that clinicians exercise caution in using compounded trilostane as any potential cost savings may be offset by the highly variable drug concentrations that have been detected within and between batches of medications obtained from compounding pharmacies.75 As noted previously, approximately 80% to 85% of cases of HC in the cat are due to pituitary disease.17,21,22 However, Cushing’s syndrome is considerably less common in cats than in dogs. The mean age of cats with PDH is reported to be around 10 years.21 Feline HC is often associated with insulin-resistant diabetes mellitus, with signs of polyuria, polydipsia, polyphagia, and weight loss. Cats with HC often have a potbellied appearance due to hepatomegaly and muscle weakness, and they frequently have thin fragile skin that tears and bruises easily. Additional signs include lethargy, generalized muscle atrophy, weakness, alopecia, and an unkempt haircoat (Figure 25-1). On routine laboratory testing, elevated alkaline phosphatase is much less frequently detected in cats with HC compared to dogs. Cats may have elevated alanine aminotransferase, hypercholesterolemia, azotemia, and minimally concentrated urine. Hyperglycemia and glycosuria are expected in cats with concurrent diabetes mellitus. No consistent complete blood count (CBC) changes have been reported in cats with HC.21,22 Tests used to screen for spontaneous HC in cats include the urine cortisol : creatinine ratio, the ACTH stimulation test, and the LDDST. It is important to note that the details of these protocols differ between dogs and cats, and readers are directed to more complete references for further information.21,22 The HDDST, endogenous ACTH levels, and abdominal ultrasound examination may be used to assist in differentiation of PDH from ADH in the cat. Because PDH is relatively uncommon in the cat, there are few case series and case reports on which to base treatment recommendations.76,77 Direct treatment of the pituitary tumor has been reported, with either surgical hypophysectomy or RT.59,78–80 Surgical bilateral adrenalectomy has also been described and was considered the treatment of choice for some time.81 However, these cats are often poor surgical candidates, they have poor healing ability, and complications are common. Laparoscopic adrenalectomy is potentially a better option for these patients because the incisions are much smaller and more likely to heal; however, this has not yet been reported in cats with PDH. Medical therapy with trilostane may be a reasonable option for these cats, based on the authors’ experience and a small number of published cases.82,83 This drug could be used as the sole therapy or to prepare cats for surgery or RT. Feline acromegaly is a disease of older cats resulting from chronic excessive GH secretion, usually from a functional somatotroph adenoma of the pars distalis of the pituitary gland.84 Feline acromegaly has historically been regarded as a rare condition; however, recent findings suggest that it may be significantly underdiagnosed. In a study of 184 diabetic cats, 59 had markedly increased IGF-1 concentrations, and acromegaly was confirmed in 17 of 18 cats that were examined by CT, MRI, or necropsy.85 These findings have prompted the suggestion that any cat with clinical features of acromegaly, including insulin resistance, should be screened for this disorder.86 Acromegaly is reported to be more common in male cats, with no apparent breed predilection, and most affected cats are middle-aged or older.87 The typical history is of insulin-resistant diabetes mellitus, with affected cats requiring 10 to 20 units of insulin per dose or more, often with inadequate control of the diabetes. This insulin resistance is due to a GH-induced postreceptor defect in the action of insulin on target cells. Affected cats remain polyuric, polydipsic, and polyphagic and continue to gain weight. Most cats with poorly regulated diabetes mellitus will lose weight, and therefore weight gain in this situation is highly suggestive of feline acromegaly. The physical changes of acromegaly develop slowly and are often not noted by the owner until they are advanced. These changes may include enlarged feet, broadening of the face, protrusion of the mandible (Figure 25-2), increased spacing between the teeth, and abdominal enlargement.85,87–90 Owners of affected cats also frequently note noisy or stertorous breathing or respiratory stridor.85,89–91 Physical examination may reveal additional abnormalities, such as enlarged abdominal organs and cardiac murmurs, arrhythmias, or a gallop rhythm.85,87,90 Neurologic signs associated with the pituitary tumor appear to be generally uncommon but may be underrecognized or underreported. Lethargy, mental dullness, or impaired vision may occur but can often be subtle. Affected cats may also demonstrate signs of diabetic neuropathy or lameness, possibly due to acromegaly-associated arthopathy.85,87 Additional complications of acromegaly may include cardiac disease and protein-losing nephropathy.87 Acromegaly is the result of excessive GH secretion from a pituitary gland tumor, and elevated GH levels have been reported in several cats with acromegaly.* Overlap between GH values in diabetic cats with and without acromegaly has also been noted.93 An ovine GH assay has been validated for the diagnosis of feline acromegaly and is available in Europe.94 This assay clearly differentiated between normal cats and cats with acromegaly. Unfortunately, at the time of writing, a feline GH assay was not reliably available in the United States. The physical changes in patients with acromegaly are due to the anabolic effects of GH, which are mediated by peripherally synthesized IGF-1.84 This hormone is predominantly produced in the liver, and levels of IGF-1 increase in the presence of chronically increased GH production. Because GH secretion may be pulsatile, even in some acromegalics, and because it has a short half-life, increased serum IGF-1 has been suggested to be a more sensitive test for acromegaly because it may reflect GH levels over the preceding 24 hours.84,86 Serum IGF-1 values are widely reported in acromegalic cats.* A recent study confirmed that IGF-1 measurement is a useful screening test for feline acromegaly, with sensitivity and specificity of 84% and 92%, respectively.89 There was no difference in serum IGF-1 concentrations among well-controlled diabetic cats, poorly controlled diabetic cats, and healthy cats.89 The highest IGF-1 noted in a diabetic cat was 153 nM, with a normal reference range of 12-92 nM; thus there is some overlap between the IGF-1 values found in acromegalic cats and those found in poorly regulated diabetic cats. However, it is these authors’ experience that IGF-1 levels in cats with acromegaly are usually at least twice the value of the high end of the reference range. Feline IGF-1 measurement is currently readily available to veterinarians in the United States, and this test should be considered in cats with diabetes mellitus that appear to be insulin resistant.86 The presence of a pituitary tumor can be demonstrated by either CT or MRI in cats with acromegaly, and both have been reported in the literature.† MRI is likely more sensitive than CT,85 but both imaging modalities may reveal a normal pituitary in a cat with acromegaly, if the size of the mass is below the limit of detection. Treatment options for acromegaly in human medicine include surgery, conventional external-beam RT, stereotactic RT (SRT), and medical therapy.103–105 Some of these therapies are also used in feline patients, but not all are available and many have not been adequately evaluated in cats. In humans, transsphenoidal surgery to remove the pituitary tumor is generally regarded as the treatment of choice106; however, there are only rare reports of the use of surgery for treatment of feline acromegaly.79,96,99 RT is rarely used as a first-line therapy in human medicine.105 In contrast, RT is the treatment for feline acromegaly that is most widely reported in the veterinary literature.* Conventional fractionated RT is administered in multiple fractions typically spread over a period of weeks. Protocols range from 5 fractions given weekly to as many as 20 fractions given over a period of 4 weeks.80,98,108 A recent study showed that RT improved diabetic control in 13 of 14 cats, although IGF-1 concentrations did not correlate with this improved control.100 Improved control of diabetes mellitus has also been noted in other studies.† There are few reports of long-term follow-up of acromegalic cats receiving RT; thus it is difficult to assess the risk of complications of this modality in this species. Published case reports and case series of cats receiving conventional RT suggest that short- and long-term adverse effects of this therapy are relatively uncommon.80,100,107,108 Disadvantages of RT as a treatment for feline acromegaly include cost, availability, and the necessity for repeated anesthetic events. The latter disadvantage can be lessened by the use of SRT (see Chapter 12). SRT is widely used in the treatment of pituitary tumors in human patients.109 There is one case series in veterinary medicine in which cats with pituitary tumors received treatment with a linear accelerator-based modified radiosurgical approach.101 Cats received a single large dose of radiation, but it was delivered in a nonconformal fashion. The technique was reported to be safe and effective. At Colorado State University (CSU), SRT is routinely used in the treatment of feline acromegaly, with promising results.110 Cats typically receive 2 to 4 fractions of radiation administered over a period of up to 5 days. This offers considerable advantages in terms of owner time commitment and the risks of anesthesia and hospitalization in elderly diabetic cats. Medical therapy for acromegaly is commonly used in humans, either as a first-line treatment or as an adjunct to surgery or RT. The classes of drugs used are somatostatin analogs, GH-receptor antagonists, and dopamine agonists.103–106 GH-receptor antagonists have not been evaluated in cats, and dopamine agonists do not appear to be useful in this species.111 Somatostatin analogs, also termed somatostatin receptor ligands (SRLs), bind to somatostatin receptors, suppressing the release of GH from the pituitary gland. The medications are available as long- or short-acting preparations, and response to SRLs is assessed by measurement of IGF-1 and GH levels and tumor size and evaluation of clinical signs.103 Octreotide has been evaluated in a small number of cats with acromegaly. In five cats, short-acting octreotide was used for up to 4 weeks, with no apparent improvement in GH levels.87,92 However, a more recent study showed that GH levels were significantly decreased for up to 120 minutes postinjection in five cats with acromegaly that received a single dose of octreotide.95 These studies used the short-acting form of octreotide and were performed over a very short time period without assessment of clinical response. A study is currently underway at CSU to evaluate the use of a long-acting octreotide preparation for the treatment of feline acromegaly. For many cats with acromegaly, insulin therapy is the only treatment that is available or acceptable to the owner. In general, these patients should receive the amount of insulin that is necessary to control their diabetes, although adequate blood glucose regulation can be difficult to achieve in many cases. The use of home blood glucose monitoring, with close cooperation between the owner and veterinarian, is strongly recommended. The feeding of a low-carbohydrate diet may also be beneficial.111 It should be expected that these patients will receive insulin doses in the range of 10 to 20 units per dose or more. Concurrent illnesses and complications of acromegaly and diabetes mellitus should also be addressed. The short-term prognosis for cats diagnosed with acromegaly is generally fair to good, but the long-term prognosis is poor. Patients may succumb to cardiac or renal failure, neurologic disease, or complications of poorly regulated diabetes mellitus.84 The median survival was reported to be 20.5 months in one case series.87 The prevalence of primary adrenal gland tumors in the dog and cat is difficult to discern from the literature. A search of the Veterinary Medical Database from 1985 to 1996 revealed that primary adrenal tumors were reported in approximately 0.17% to 0.76% of pet dogs (representing 1% to 2% of all canine tumors) and 0.03% of cats (representing 0.2% of feline tumors).112 However, this likely underestimates the true prevalence because gross pathologic or histopathologic records were not available for the majority of these patients. For dogs and cats undergoing necropsy or adrenal surgery, it appears that tumors of the adrenal cortex are more common than those of the medulla. A retrospective study of patients with adrenal tumors identified from surgical biopsies or necropsy during a 20-year period at the University of California, Davis (UC Davis), demonstrated that 195 (41%) of 472 neoplastic canine adrenal lesions were adrenocortical tumors (154 adenomas; 41 carcinomas), 151 (32%) were pheochromocytomas (84 benign; 67 malignant), and 126 (27%) were metastatic lesions.113 Of 20 feline adrenal neoplastic lesions, 6 (30%) were adrenocortical tumors (3 adenoma; 3 carcinoma), 2 (10%) were pheochromocytomas (1 benign; 1 malignant), and 12 (60%) were metastatic lesions.113 Less than half of these metastatic lesions were grossly visible at necropsy. Lymphoma was the most common cancer to spread to the adrenal glands in both species. Other metastatic tumors commonly identified in the dog included hemangiosarcoma, mammary carcinoma, histiocytic sarcoma, pulmonary carcinoma, and melanoma. Right and left adrenal glands were affected equally, as were the cortex and medulla. The only notable exception was that all metastatic melanomas were restricted to the adrenal medulla.113 A number of case series in the last decade have documented the outcome of adrenal surgery in dogs.43,45,114,115 When the data from these cases are combined, a histopathologic diagnosis was reported for a total of 191 adrenal tumors, with 153 (80%) arising from the adrenal cortex and 33 (17%) from the medulla. The remaining tumors included two myelolipomas and one each of fibrosarcoma, lymphoma, and leiomyosarcoma. For the adrenocortical tumors that were further classified, 63 of 125 (50%) were carcinomas, 54 of 125 (43%) were adenomas, and 8 of 125 (6%) were described as hyperplastic lesions. It is important to note the bias inherent in these data because only dogs that underwent surgery are included. Advanced imaging techniques such as ultrasonography, CT, and MRI have now greatly enhanced our ability to identify both clinical and subclinical adrenal abnormalities,38,43,116,117 and it appears that the adrenal gland is affected with neoplasia more commonly than was previously suspected. The ability to detect these adrenal lesions also leads to diagnostic dilemmas as the clinician attempts to elucidate whether the lesions arise from the cortex or medulla, whether they are functional or nonfunctional, and whether they are benign or malignant. Functional adrenal tumors may secrete cortisol, catecholamines, aldosterone, sex hormones, or steroid hormone precursors, and these may be associated with specific clinical and laboratory findings. Hormonal testing and imaging techniques are central to the diagnostic evaluation of these patients so that the most appropriate course of therapy can be pursued. Large adrenal masses may be detected on abdominal radiographs,34,118,119 and the presence of mineralization suggests an adrenal tumor; however, this finding is not highly specific, and it cannot be used to differentiate between benign and malignant masses. The normal ultrasonographic appearance of canine adrenal glands has been described,116,120 and there are many reports of the ultrasonographic appearance of adrenal lesions in dogs. Abdominal ultrasound examination is frequently used to detect metastatic disease and determine the local invasiveness of adrenal tumors. Ultrasonography has been reported to be 80% to 100% sensitive and approximately 90% specific for the detection of adrenal tumor invasion into the caudal vena cava.43,45 The CT appearance of both normal and abnormal canine adrenal glands has been described.46,119,121–124 Contrast-enhanced CT has been shown to provide accurate preoperative evaluation of canine adrenal masses, with 92% sensitivity and 100% specificity for the detection of vascular invasion by adrenal tumors.125 The MRI appearance of presumed normal canine adrenal glands has also been described,126 but as yet there are few reports that document the systematic use of MRI for evaluation of adrenal lesions in dogs and cats. The characterization of adrenal lesions by imaging techniques has undergone recent significant advancement in human medicine. Malignant and benign lesions can frequently be differentiated using modalities such as contrast-enhanced ultrasound, CT densitometry, CT washout characteristics, chemical shift MRI, positron emission tomography (PET), and PET/CT.127–130 Most of these techniques have yet to be explored in veterinary medicine. Functional cortisol-secreting tumors of the adrenal cortex are responsible for 15% to 20% of canine and feline cases of naturally occurring HC, with PDH accounting for 80% to 85%.17,18,21,22 In the necropsy and surgical biopsy data from UC Davis, adrenocortical adenomas were almost 4 times more common than carcinomas113; however, the functionality of these tumors was not assessed. A review of case reports of functional adrenocortical tumors in dogs suggests that approximately 60% of surgically removed tumors are carcinomas.* Adenomas are typically smaller, with tumors larger than 2 cm more likely to be carcinomas.132 However, adenomas up to 6 cm have been reported.17 On histopathologic examination, adenocarcinomas appear more likely to exhibit a trabecular growth pattern, peripheral fibrosis, capsular invasion, necrosis, and/or hemorrhage.132 They are less likely to exhibit cytoplasmic vacuolization, extramedullary hematopoiesis, or fibrin thrombi. Approximately 20% of adrenocortical carcinomas locally invade into the phrenicoabdominal vein, with extension into the renal vein and/or caudal vena cava.42,131 Intravascular invasion has the potential to cause severe and life-threatening intraabdominal or retroperitoneal hemorrhage.45,133 Metastasis was identified in approximately 50% of dogs with adrenocortical carcinomas.131,132 Although involvement of the liver and lungs is most common, other organs reported to be affected with metastases include the kidney, ovary, mesenteric lymph nodes, peritoneal cavity, and thyroid gland. In the absence of evidence of tumor invasion or metastasis, there are no consistent clinical, biochemical, or imaging findings that reliably distinguish between functional adrenocortical adenomas and carcinomas in dogs and cats. The cellular and molecular events underlying the development of canine adrenocortical tumors are unknown. Recent studies have demonstrated downregulation of ACTH receptors in cortisol-secreting adrenocortical carcinomas and ectopic expression of gastric-inhibitory polypeptide and vasopressin (2) receptor proteins in neoplastic zona fasciculata tissue from canine adrenocortical tumors.134,135 The significance of these findings in tumorigenesis remains to be elucidated. Dogs with PDH and dogs with ADH are similar in age, but almost 50% of dogs with ADH weigh more than 20 kg, compared to approximately 25% of dogs with PDH.34 The historic features, physical changes, clinical signs, and basic laboratory findings in canine Cushing’s syndrome are essentially the same in dogs with PDH and dogs with ADH and are described in the earlier section on Pituitary Corticotroph Tumors. Similar screening tests are used to confirm the diagnosis of HC; however, the sensitivity of the ACTH stimulation test for the diagnosis of ADH is only around 60%.32 Thus the LDDST is a better screening test when ADH is suspected. Dogs with ADH fail to show suppression on the LDDST or the HDDST, and differentiation from PDH is generally determined by imaging studies, particularly abdominal ultrasound examination,33 and measurement of endogenous ACTH levels.34 Excessive secretion of glucocorticoids by a functional adrenocortical adenoma or adenocarcinoma occurs independent of pituitary control, with secondary atrophy of the normal adrenocortical cells in both the affected and contralateral adrenal glands. Unfortunately the functional atrophy of the contralateral adrenal gland is not always easily detected on abdominal ultrasonography.39 This finding, termed equivocal adrenal asymmetry, is also observed in some dogs with PDH, associated with asymmetric hyperplasia of the glands.37 The results of a recent ultrasound study of dogs with equivocal adrenal asymmetry suggested that a maximal dorsoventral thickness of the smaller gland of less than 5.00 mm was consistent with a diagnosis of ADH.40 Surgical adrenalectomy is the treatment of choice for dogs with ADH,* and surgical management is further addressed later. An early case series of dogs undergoing surgical removal of adrenocortical tumors revealed that 60% of patients were euthanized during surgery or died within 2 weeks.131 In a more recent case series, the perioperative mortality has ranged from 19% to 28%.17,42,43,136 In one series of 144 dogs undergoing surgical removal of a functional adrenocortical tumor, the prognosis was described as excellent for patients that survived 4 weeks postoperatively, with an average life expectancy of 3 years. Nine of 144 dogs were euthanized at the time of surgery, and 29 dogs died during surgery or immediately postoperatively.17 Median survival times of 230 to 778 days have been reported for dogs undergoing adrenalectomy for adrenal carcinomas,42,114,115 compared to a MST of 687.5 days for dogs with adenomas.114 Laparoscopic adrenalectomy has been described for noninvasive adrenocortical carcinomas in a small number of dogs; further studies are needed to determine if this technique can reduce the postoperative complications associated with conventional surgery.137 Medical therapy for ADH should be used when surgery is not a good option for the patient or client, or it may be used prior to adrenalectomy in patients that are significantly debilitated by HC. The primary options for medical therapy are mitotane and trilostane. Treatment with mitotane as an alternative to surgical adrenalectomy utilizes the drug as a true cytotoxic agent. Detailed protocols are readily available,138 and clinicians should be aware that this approach typically requires higher doses of mitotane than those used in PDH139 and that relapses are common. However, this treatment can be effective, with a mean survival of 16.4 months reported in a series of 32 dogs. Dogs without evidence of metastatic disease may show a better response to mitotane therapy.140 Trilostane is not a cytotoxic drug, but it has been used to successfully manage patients with ADH,68,69,141 including a small number of dogs with metastatic disease.142 A recent retrospective study comparing trilostane and mitotane in dogs with ADH reported a median survival of 353 days with trilostane therapy compared to 102 days for mitotane. These survival times were not significantly different; however, this study did further confirm that survival times are significantly decreased in the presence of metastatic disease.143 Functional adrenocortical tumors in dogs and cats can also secrete one or more sex hormones, including androstenedione, progesterone, 17-hydroxyprogesterone, testosterone, and estradiol. These tumors may or may not secrete glucocorticoids, and some patients have been reported to show signs of HC in the absence of elevated cortisol levels on typical screening tests.144–149 Signs of sex hormone excess appear uncommon in dogs with sex-hormone–secreting adrenal tumors but have been reported in a small number of cats.150,151 Aldosterone-secreting adrenocortical tumors have rarely been reported in dogs,152–154 but there is increasing evidence that primary hyperaldosteronism (also termed primary aldosteronism or Conn’s syndrome) may be an underrecognized condition in cats. In fact, it has been suggested to be the most common adrenocortical disorder in this species.155 Affected cats are middle-aged or older, and the most common clinical sign is muscle weakness due to hypokalemia. Arterial hypertension is frequently detected in these patients and may be associated with ocular changes. Routine laboratory testing often reveals hypokalemia, but hypernatremia is uncommon, presumably due to intact water balance mechanisms in these patients. Some cats may also have evidence of concurrent renal disease. Plasma aldosterone can be measured in cats, and normal or elevated levels in the face of hypokalemia would be regarded as inappropriate. However, definitive diagnosis using aldosterone levels is difficult without the measurement of plasma renin activity and the calculation of an aldosterone : renin ratio.156 Unfortunately, a plasma renin activity assay is not readily available to most clinicians. An oral fludrocortisone suppression test has recently been suggested to be a useful diagnostic test for feline hyperaldosteronism,157 but the use of this tool has not yet been widely reported. Imaging of the adrenal glands is often performed in the evaluation of these patients,158,159 and this may distinguish between unilateral and bilateral lesions and also reveal the presence of vascular invasion or metastatic disease. Most cats with hyperaldosteronism have an adrenal adenoma or carcinoma.160 Bilateral adenomas have been reported,160 and some cats have adrenal hyperplasia.156 Adrenalectomy is the treatment of choice for cats with unilateral disease, and good outcomes have been reported for both adenomas and carcinomas, as well as for tumors associated with vena cava thrombosis.160–163 Medical management with potassium supplementation, antihypertensive drugs, and the aldosterone antagonist spironolactone can give reasonable survival times in patients that are not surgical candidates.156,160,161 Chromaffin cells are part of the sympathetic nervous system and are present in the adrenal medulla and other locations throughout the body. Neoplastic chromaffin cells in the adrenal medulla give rise to pheochromocytomas, which are tumors that predominantly secrete catecholamines. Chromaffin cell tumors (termed paragangliomas or extraadrenal pheochromocytomas) can arise in other parts of the body, but these are rare in veterinary medicine. Pheochromocytomas are uncommon in dogs and rare in cats.164,165 In past decades, the diagnosis of pheochromocytoma was most often made incidentally at necropsy,166,167 but these tumors are now likely to be detected antemortem because advanced abdominal imaging techniques are routinely used in small animal patients. Pheochromocytomas are generally considered to be malignant tumors in dogs.164 Metastasis is reported in up to 40% of affected dogs; sites include liver, spleen, lung, regional lymph nodes, bone, and CNS.166–168 Vascular invasion by the tumor has been reported in as many as 82% of cases.43,45,169 This finding is not specific for pheochromocytoma because vascular invasion can also occur with adrenocortical tumors. Pheochromocytoma is usually diagnosed in older dogs,164,167 and males may be overrepresented.169 Catecholamine release by pheochromocytomas is typically episodic, and thus clinical signs may be intermittent and often absent at the time of physical examination. Signs may include weakness, episodic collapse, panting, anxiety, restlessness, exercise intolerance, decreased appetite, weight loss, polyuria, and polydipsia. Physical examination of dogs with pheochromocytoma may be normal due to the episodic nature of catecholamine release or may reveal tachypnea, panting, tachycardia, weakness, pallor, cardiac arrhythmias, or hypertension.164,166,167 Some dogs have signs referable to an abdominal mass, and acute collapse may occur secondary to tumor rupture with abdominal or retroperitoneal bleeding.133 There are no consistent abnormalities on the CBC, serum biochemistry profile, or urinalysis in dogs with pheochromocytomas. Diagnostic imaging, particularly abdominal ultrasound examination, is central to the evaluation of patients with pheochromocytoma. In many dogs, evaluation for pheochromocytoma occurs after an adrenal mass is found when abdominal ultrasonography is performed for other reasons. In addition to revealing the presence of an adrenal tumor, abdominal ultrasonography may reveal metastatic disease and is sensitive and specific for detecting vascular invasion by adrenal tumors.43,45 CT and MRI are the imaging modalities of choice for humans with pheochromocytomas, and early experience in canine patients has been encouraging.170–172 Abdominal radiographs may reveal the presence of a large adrenal mass166,170 but are generally less informative than ultrasound examination. Thoracic radiographs are recommended to evaluate the cardiovascular system and for detection of pulmonary metastases in any patient with a suspected adrenal tumor. There are rare reports of PET or nuclear scintigraphy imaging in dogs with pheochromocytomas.173,174 Immunohistochemical staining for chromogranin A can distinguish pheochromocytomas from adrenocortical tumors on tissue obtained at surgery or necropsy.175 Plasma and urinary concentrations of catecholamines and their metabolites are routinely measured in humans for the diagnosis of pheochromocytoma.164 Recent reports have suggested that urinary catecholamine and metanephrine to creatinine ratios hold promise as a diagnostic tool in dogs.176–178 Plasma-free metanephrine and normetanephrines have also been evaluated in cats and dogs, with encouraging preliminary results.165,179 Surgery is the only definitive treatment for pheochromocytoma.43,164,166,167,169 This should be performed by an experienced surgical and anesthesiology team because potentially life-threatening complications, including hypertension, hypotension, cardiac arrhythmias and hemorrage,169 may occur during anesthetic induction and handling of the tumor. It has been shown that dogs receiving phenoxybenzamine, a noncompetitive α-adrenergic antagonist, prior to surgery are significantly more likely to survive adrenalectomy. Specifically, dogs that received this medication at doses ranging from 0.1 to 2.5 mg/kg every 12 hours, for a median period of 20 days, had a 13% mortality rate, compared to a mortality rate of 48% in dogs that did not receive this therapy.169 Chemotherapy and RT have not been evaluated in dogs with pheochromocytoma. RT using 131I-metaiodobenzylguanidine (131I-MIBG) was recently reported in one case.180 The prognosis for dogs with pheochromocytoma is affected by tumor size, presence of metastases, and local invasion. A MST of 374 days has been reported after surgical treatment of pheochromocytoma,114 and some dogs may survive for as long as 2 to 3 years.167,181 Dogs without metastatic disease that survive the perioperative period appear to have a good prognosis.164 Prior to adrenalectomy, every attempt should be made to determine whether an adrenal tumor is functional, whether there is evidence of metastatic disease, and whether there is vascular invasion. Patients suspected to have a pheochromocytoma should be treated preoperatively with phenoxybenzamine. If tachyarrhythmias are present, a β-blocker such as propranolol or atenolol may also be administered but should only be started after α-adrenergic blockade has been initiated to prevent unopposed α-adrenergic stimulation and severe hypertension.164 Patients with HC due to ADH may be medically managed with trilostane or mitotane prior to surgery if they are significantly debilitated by their disease, although this is rarely necessary. The potential for intraoperative and postoperative complications associated with adrenalectomy is significant182; these cases are best managed by an experienced team, including a surgeon, anesthesiologist, internist, and critical care specialist. Particular concerns with pheochromocytomas include cardiovascular complications and hemorrhage, as noted previously. Patients with functional adrenocortical tumors are at risk for adrenocortical insufficiency, pulmonary thromboembolism, pancreatitis, renal failure, and wound dehiscence.* Protocols are available to guide the perioperative management of adrenalectomy patients.17 At CSU, patients with pheochromocytoma are treated preoperatively with phenoxybenzamine as described earlier. Patients with functional adrenocortical tumors receive heparin and corticosteroid therapy during and after surgery. Postoperatively, an ACTH stimulation test is performed 24 to 48 hours after surgery, and electrolytes and blood glucose are measured frequently. The duration of prednisolone therapy and the necessity for mineralocorticoid supplementation are each determined on an individual case basis. For patients with adrenal tumors of unknown origin, preoperative phenoxybenzamine is recommended, and the protocol for functional adrenocortical tumors is followed, until postoperative ACTH stimulation test results and histopathology results are available to guide further management. The overall perioperative mortality rate for dogs undergoing adrenalectomy for all adrenal tumors is around 10% to 20%,43,45,114,115 and MSTs of 690 to 953 days have been reported.114,115 A number of investigators have evaluated prognostic factors and predictors of outcome in these patients. In two studies, the presence of caval tumor thrombus did not affect perioperative morbidity and mortality, although the long-term prognosis for dogs with an adrenocortical tumor may be poorer in the presence of a thrombus.43 In contrast, a more recent study suggested that vein thrombosis was associated with a poorer prognosis.115 In the latter study, vein thrombosis was associated with tumors with major axis length of 5 cm or larger, and the presence of metastases or tumor size of 5 cm or larger were both associated with a poorer prognosis.115 Large tumors were also associated with increased perioperative mortality in a series of dogs undergoing elective or emergency adrenalectomy. The dogs in this report that had emergency surgery for acute adrenal bleeding experienced a 50% perioperative mortality rate.45 Advances in abdominal imaging have led to the diagnostic dilemma of the incidental adrenal mass (“incidentaloma”) in both human and veterinary medicine. When an incidental adrenal mass is identified in a dog or cat, a thorough history and physical examination, including blood pressure measurement and fundic examination, are indicated. Endocrinologic testing should be pursued to rule out a functional adrenocortical tumor. Given the high incidence of metastatic lesions in canine and feline adrenal glands, imaging of the thorax and abdomen should be performed to rule out another primary tumor. Aspiration cytology and ultrasound- or CT-guided biopsies are not routinely recommended for incidentalomas because of the high risk of complications and the inability to reliably differentiate benign and malignant lesions.38,112 Adrenalectomy should be considered for masses that are functional, locally invasive, or larger than 2.5 cm in maximum dimension. Masses smaller than 2 cm with no evidence of hormonal activity should be monitored with abdominal ultrasonography. A suggested interval is to repeat the sonogram 1 month after the initial study and then after 2, 4, and 6 months, with further intervals determined by the appearance of the mass and the clinical status of the patient.164 Thyroid tumors account for 1.1% to 3.8% of all tumors in dogs.183–185 In necropsy studies, it is estimated that 30% to 50% of canine thyroid tumors are benign adenomas.183,186 A recent Veterinary Medical Database review reported that 90% of 545 canine thyroid cancers submitted to the database from veterinary teaching hospitals were carcinomas or adenocarcinomas, with 9.3% being adenomas.185 Most adenomas are small, noninvasive, and clinically silent. Consequently, almost all canine thyroid masses associated with clinical signs are malignant.184,187,188 Thyroid tumors of follicular cell origin are subclassified as papillary, follicular, compact (solid), or anaplastic. All subgroups stain positive for thyroglobulin and thyroid transcription factor-1.189–191 Papillary carcinomas are most common in humans,10,192 whereas follicular and compact forms are most common in dogs.1,184,189,193 Medullary thyroid carcinomas, also called parafollicular or C-cell carcinomas, are relatively uncommon in both humans and dogs.191 Positive immunohistochemical staining for calcitonin is the most accurate way to identify these tumors, but they also often stain positive for calcitonin gene-related peptide, thyroid transcription factor-1, chromogranin A, and neuron-specific enolase.175,189–191,194 The etiology of thyroid neoplasia in dogs is largely unknown. The molecular pathogenesis of thyroid neoplasia is best defined in humans.5,7,10 The classic hypothesis involves a discrete series of mutations. Activation of receptor tyrosine kinases such as RET and TRK are common in papillary carcinomas, activating mutations in RAS are frequently identified in follicular carcinomas, and inactivation of p53 is commonly seen in anaplastic carcinomas. Thyroid-stimulating hormone (TSH) or the TSH receptor may play a contributing role in carcinogenesis.195 The TSH receptor in humans with thyroid neoplasia is frequently affected with either hyperfunctioning or silencing mutations. Canine thyroid tumors retain TSH receptors, and hypothyroid beagles that did not receive thyroid hormone supplementation had an increased incidence of thyroid tumors, presumably due to TSH trophic effects without feedback in the context of potential mutations.196,197 Thyroid irradiation is associated with an increased incidence of thyroid tumors in all species, including humans, rodents, and dogs.1,10,186,192 In dogs, one report identified a p53 mutation in 1 of 23 primary thyroid carcinomas.198 Another report confirmed trisomy 18 in a canine thyroid adenoma.199 Thyroid tumors typically arise in older dogs, with a median reported age of 9 to 11 years.184,193,200–202 A sex predilection has not been reported. Predilection of breeds for thyroid tumors includes golden retrievers, beagles, Boxers, and Siberian huskies.184,185 Familial medullary thyroid carcinoma has also been described in a family of dogs with an Alaskan malamute influence.203 The right and left lobes are affected with equal frequency in canine thyroid tumors, and as many as 60% of patients will have bilateral involvement.200,204 On rare occasions, ectopic thyroid tissue can give rise to tumors at the base of the tongue, cervical ventral neck, cranial mediastinum, and heart.204–210 Up to 35% to 40% of dogs have visible metastatic disease at initial presentation, and as many as 80% will ultimately develop metastasis.184,191,204,211 Metastatic potential is reported to increase when the primary tumor volume exceeds 23 cm3 and approaches 100% when tumor volume exceeds 100 cm3.186 Bilateral tumors are 16 times more likely to metastasize than unilateral tumors.200 The lungs and regional lymph nodes, including the retropharyngeal, cranial cervical, and mandibular lymph nodes, are affected most commonly, but a wide variety of tissues can be affected. Medullary carcinomas may have a lower metastatic potential than follicular and solid carcinomas.191 The majority of canine thyroid carcinomas are nonfunctional. Based on clinical signs and serum T4 concentrations, approximately 60% of patients are euthyroid, 30% are hypothyroid secondary to destruction of the normal thyroid parenchyma, and 10% are hyperthyroid.188,202,212–214 Most dogs are presented for a palpable ventral cervical mass.184,191,193 Less common abnormalities include coughing, rapid breathing, dyspnea, dysphagia, dysphonia (change in bark), laryngeal paralysis, Horner’s syndrome, and facial edema. Acute severe hemorrhage can occur secondary to invasion into the cervical vasculature.215 In addition to clinical signs referable to the physical thyroid mass, dogs with hyperthyroidism frequently exhibit polyphagia, weight loss, muscle wasting, polyuria, and polydipsia.188,212–214 The differential diagnosis for a mass in the region of the thyroid gland in dogs includes abscesses or granulomas, salivary mucoceles, lymphatic metastasis from tonsillar squamous cell carcinoma, lymphoma, carotid body tumor, and sarcomas. In humans, thyroid cytology is very accurate for identifying thyroid tumors and distinguishing whether they are benign or malignant.10,192 Accuracy of cytology in dogs with thyroid masses is reported to be problematic. In several reports, cytology confirms the mass to be of thyroid origin in only half of affected dogs, and definitive recognition of malignancy occurs less often.184,188 Use of a needle without physical aspiration and thorough examination of the feathered edge may improve diagnostic accuracy. Malignant thyroid tumors have a higher vascular density than normal thyroid tissue and benign tumors,202 and hemodilution is a common problem (see Chapter 6). This increased vascularity also adds significant risk to large core needle biopsy procedures. Routine staging for dogs with thyroid carcinoma includes general health assessment with laboratory evaluation (CBC, serum biochemistry profile, and urinalysis), three-view thoracic radiographs, and cytologic or histologic evaluation of the mandibular lymph nodes. Cervical ultrasonography can be used to confirm if a mass is of thyroid origin and to assess invasiveness and vascularity.216,217 The retropharyngeal and cranial cervical lymph nodes can also be examined for evidence of metastasis. The MRI and CT appearances of the normal canine thyroid have been described,218,219 and these modalities are useful in the investigation of cervical masses in the dog and in the staging of thyroid carcinomas.215,218,219 Scintigraphy using 99mTc-pertechnetate or, less commonly, radioactive iodine (123I or 131I) is performed primarily to identify local residual disease after surgery, ectopic tumors, or metastatic disease.* Most primary tumors are visualized, although the pattern of uptake is often heterogeneous. Metastatic disease is identified less consistently. To be visualized with scintigraphy, a thyroid tumor must be capable of trapping 99mTc-pertechnetate or trapping and organifying 123I or 131I. It may or may not be able to complete the remaining steps necessary for synthesis and secretion of functional thyroid hormone. Treatment of canine thyroid carcinomas is dictated by the size of the mass, extent of invasion, presence or absence of gross metastatic disease, and any concurrent symptoms of thyrotoxicosis. Surgical excision provides the best outcome with the least morbidity when tumors are freely movable without extensive deep tissue invasion.191,193,222 Thyroidectomy is not recommended when the tumor is not freely movable in all directions or extensively invades adjacent structures, including major vasculature, recurrent laryngeal nerves, the vagosympathetic trunk, the larynx, the trachea, or the esophagus. Extensive hemorrhage can result from the vascularity of the tumor, invasion into adjacent blood vessels, and local coagulopathies.202 Other potential complications of thyroidectomy include hypocalcemia due to hypoparathyroidism if the parathyroid glands are removed, damage to the recurrent laryngeal nerve(s), and hypothyroidism after bilateral thyroidectomy.193,223 According to limited, retrospective data sets, it was estimated that only 25% to 50% of thyroid carcinomas were mobile and amenable to surgery at the time of initial diagnosis.191,193 Palpation under anesthesia and preoperative imaging are current general recommendations for surgical evaluation at the authors’ institution, with some extracapsular extension and invasion considered acceptable. Median survival after thyroidectomy is around 3 years if the tumor is freely movable and 6 to 12 months if the tumor is more invasive.191,193 Recently, a report describing resection of mobile, discrete bilateral thyroid tumors in a limited number of dogs suggested reasonable overall survival with persistent postoperative management of thyroid and parathyroid endocrinopathies.224 Nonresectable thyroid carcinomas may be managed with radiation as a primary therapy or as a means to achieve a surgical option. External-beam RT is used most commonly for dogs. One study evaluated definitive RT (48 Gy delivered in 4 Gy fractions on an alternate-day schedule) in 25 dogs with unresectable thyroid carcinomas and no visible metastasis.200 Tumors either stabilized or decreased in size. The time to maximal tumor reduction ranged from 8 to 22 months in dogs whose tumors did respond. The progression-free survival rates were 80% at 1 year and 72% at 3 years. The first cause of treatment failure was local progression in three dogs, metastasis in four dogs, and concurrent local progression and metastasis in three dogs. Limited information exists regarding the use of definitive radiation in the adjuvant or neoadjuvant settings.201,225 This same RT protocol described was evaluated in an additional eight dogs, seven of which had undergone incomplete thyroidectomy prior to irradiation.201 Median survival was just over 2 years (range 1 to 3 years). None developed local recurrence, although four died from metastatic disease. Radiation-induced toxicoses to the larynx, trachea, and esophagus are usually well tolerated. Hypothyroidism may develop months to years after treatment.200,225 For dogs that present with gross metastatic disease, hypofractionated RT may still provide effective palliation of the primary tumor, due to a generally slow rate of progression in both the primary and metastatic lesions. In a study evaluating palliative RT as the sole treatment modality, 13 dogs received 36 Gy in four weekly 9 Gy fractions.211 Complete or partial reduction of the primary tumor occurred in one and nine dogs, respectively. Local progression occurred in five dogs, 11 to 24 months after irradiation. Gross metastatic disease was present in five dogs at initial presentation and developed in an additional two dogs during the study. Overall median survival was around 22 months, with local and metastatic progression occurring equally in all dogs. In humans with well-differentiated thyroid carcinoma, 131I is routinely administered postoperatively to destroy occult microscopic local or metastatic carcinoma.10,192 Experience with 131I thyroid ablation in dogs is substantial, with two recent, relatively large clinical studies providing evidence of efficacy of 131I for advanced unresectable, metastatic, or residual thyroid neoplasia.226,227 Collectively, from all reports, over 80 dogs with stage II (2 to 5 cm diameter; fixed or unfixed), stage III (>5 cm diameter, fixed or unfixed), or stage IV metastatic thyroid carcinoma have received 131I for the intent of managing tumor burden and clinical signs. MSTs for stage II and III patients exceed 2 years, and dogs with metastatic carcinoma experienced survival times of approximately 1 year. Such results are comparable to external-beam RT. Interestingly, similar survival times were noted in dogs that were hypothyroid, euthyroid, or hyperthyroid. Recommendations for 131I dosimetry remain unresolved. Fatal myelosuppression was observed in three dogs in one report, although no specific dose-effect relationship was defined.227 The biologic effect both on tumor and normal tissue is a complex function of 131I uptake that depends on extent of the tumor burden, degree of organification and excretion of 131I, bone marrow sensitivity, and administered dose of radiation. Dosing regimens are currently empiric in dogs but could be more carefully defined by administering a 131I tracer for calculation of definitive dosing, as in humans. Until such time as dosing is individualized, the maximum dose administered should be 0.2 GBq/kg (5 mCi/kg), and bone marrow monitoring posttreatment is recommended. Additional doses may be administered if necessary, as determined by persistent hyperthyroidism or activity on posttreatment nuclear scans. Dogs receiving 131I will require thyroid hormone supplementation. Chemotherapy has been evaluated in dogs with thyroid tumors. Of those dogs treated with either doxorubicin or cisplatin, 30% to 50% demonstrated a partial response (>50% reduction in volume).228,229 Individual responses have also been reported using mitoxantrone or actinomycin D.230,231 Chemotherapy may be considered for dogs with large nonresectable primary tumors and/or gross metastatic disease.
Tumors of the Endocrine System
Pathogenesis of Endocrine Tumors
Pituitary Tumors
Pituitary Corticotroph Tumors: Hypercortisolism (Hyperadrenocorticism or Cushing’s Syndrome)
Clinical Findings and Diagnostic Evaluation in Dogs
Treatment of Canine Pituitary-Dependent Hypercortisolism
Surgery
Radiation Therapy
Medical Therapy
Feline Pituitary-Dependent Hypercortisolism
Pituitary Somatotroph Tumors (Feline Acromegaly)
Adrenal Gland Neoplasia
Adrenocortical Tumors
Adrenal Medullary Tumors
Surgical Management of Adrenal Tumors
Incidental Adrenal Masses
Thyroid Gland Neoplasia in Dogs
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree