Chapter 22. Tumors of the Abdominal Cavity
SECTION A Stomach Tumors
Leslie E. Fox
Incidence/Mortality
Gastric tumors are uncommon and comprise less than 1% of all neoplasms in dogs and cats. 1-4 The most common malignant gastric tumor in dogs is adenocarcinoma (ACA). Other malignancies affecting the stomach include leiomyosarcoma (LMS), gastrointestinal stromal tumor (GIST), lymphoma, mast cell tumor (MCT), extramedullary plasmacytomas, scirrhous carcinoma, histiocytic sarcoma, and others. 1,3,5 Non-retrovirus–associated lymphoma is the most common gastric tumor in cats followed by LMS, ACA, MCT, extramedullary plasmacytomas, carcinoid, GIST, and others. 2,4,6-11 Benign lesions include gastric adenoma, adenomatous polyps, and leiomyoma. 2,3,9,12 Carcinoids are very rare neuroendocrine tumors of the gastrointestinal tract that are reported only as single case reports in dogs and cats. 13,14 Most dogs and cats with gastric tumors are geriatric (average, 10 years old). Dogs with leiomyoma are usually older (average, 16 years old), and dogs with LMS tend to be younger (7 years old). 1-3,5,7,9,12,15 Gastrointestinal lymphoma and MCT are typically diffuse within both the stomach and intestines in cats and dogs. They are discussed in Chapter 25, Section A and Chapter 23, Section D , respectively.
Etiology/Risk Factors
Breed predilections for ACA include Chow Chows, Rough Collies, Staffordshire terriers, Belgian shepherds, and Norwegian Lundehunds. 1,3,16-18 In most reports, male dogs are more frequently affected. 1,3,17 In general, cats do not appear to have breed or gender predilection. 4,6,7,9,10 Recently, the simultaneous occurrence of gastric ACA was diagnosed in two related Persian cats living in the same household. 16 Dietary factors contribute to the development of stomach cancer in humans and may play a similar role in dogs. 19 Gastric ACA may be experimentally induced in dogs by chronic feeding of nitrosamines. 20 It has been hypothesized that the association found between Helicobacter heilmannii infection in pet cats and lymphoblastic or lymphocytic lymphoma of mucosa-associated lymphoid tissue is similar to H. heilmannii –associated gastric lymphoma in humans, which resolves when the infection is eradicated with antimicrobial therapy. 21,22
Clinical Features
Clinical signs develop over weeks to many months and include chronic vomiting, anorexia (often the first clinical sign observed), lethargy, and weight loss. 1,3,5-7,9,10 Additional signs may include abdominal discomfort, ptyalism, anemia, hematemesis, and melena; they may reflect the presence of metastatic disease in other organs. Cats and dogs with gastric polyps are often asymptomatic. 13,23 Physical examination may be unremarkable except for weight loss or may include a palpable abdominal mass, pale mucous membranes, and pain on abdominal palpation. Differential diagnosis for chronic vomiting, inappetence, and weight loss are gastritis, fungal or inflammatory granulomas, foreign body, gastric ulceration, motility disorders, malignant and benign tumors, and pyloric outflow obstruction or dysfunction. Clinicopathologic data are generally normal or have non-specific changes. A non-regenerative, microcytic, hypochromic anemia resulting from chronic blood loss may occur, whereas persistent vomiting may alter electrolytes. Paraneoplastic hypoglycemia has been reported in dogs with gastric leiomyoma and LMS. 24,25
Diagnosis and Staging
Stomach cancer should be suspected in a geriatric dog or cat with weight loss, chronic vomiting, and inappetence. Survey abdominal radiography with or without contrast is often unremarkable and is considered relatively insensitive for detecting gastric wall changes. 23,26,27 When present, abnormalities include alteration in normal stomach axis (should be parallel to the 10th or 11th ribs in dogs), distention with fluid, masses and/or filling defects, calcified masses, and delayed gastric emptying (>10–12 hours in dogs). 26 Thoracic radiographs should be evaluated for complete staging, but they are typically normal.
Ultrasound examination is preferred for evaluation because stomach wall thickness, gastric lumen structure, focal motility abnormalities, lumen contents, and regional lymph nodes are visualized rapidly, in real time, without special patient preparation or positioning. 27-29 Loss of normal five-layer gastrointestinal tract wall structure and/or wall thickness greater than 7 mm, and decreased wall motility (<4-5 contractions/minute) are consistent with gastric wall infiltration, although confirming the cause (necrosis, neoplasia, hemorrhage, edema, inflammation) requires histopathologic examination. 26 Ultrasound-guided, percutaneous fine-needle aspiration (FNA) cytology, or needle tissue biopsy can be performed with minimal invasiveness. 27-29 Cytologic examination of a FNA sample may support a diagnosis of gastric neoplasia, particularly if the slide is appropriately prepared and the tumor exfoliates well, such as with lymphoma, MCT, or some ACAs. 28 Hypoechoic and hyperechoic lesions in the liver and spleen and enlarged locoregional lymph nodes are consistent with metastatic tumor; however, normal-sized gastric lymph nodes should be evaluated microscopically for metastasis. 23,26
Endoscopy is minimally invasive and allows direct visualization and sampling of the mucosa. The gastric wall can be diffusely affected and look normal; therefore, multiple biopsy samples are needed, regardless of the mucosal appearance. Gastric lesions may appear diffusely infiltrative or localized and discrete. 1,3,26,27 They may be smooth or ulcerative, proliferative, and/or polypoid. 1,3,26,27 Generally, ACAs are highly invasive, often through the serosa, and are proliferative and ulcerative, whereas adenomatous polyps, leiomyomas, and LMSs are typically covered with intact mucosa. 1 Carcinomas are typically located in the lesser curvature or pyloric antrum, whereas smooth muscle tumors are found in the area of the cardia. 1,3 Peritumoral inflammation, food retention, and necrosis associated with ulceration make definitive histologic diagnosis from mucosal pinch biopsies difficult; however, sampling at the edges of the lesion optimizes tumor identification. Abdominal exploratory may be needed to assess the extent of disease and obtain sufficient tissue (full-thickness biopsy) for diagnosis.
Metastasis
Because ACAs are often detected late in the course of the disease, the locoregional metastatic rate is greater than 70% to 80%. 1,3,17 Metastasis may be through the lymphatic route and/or tumor cell exfoliation directly into the abdominal cavity. Carcinomas metastasize to the gastric lymph nodes (most often affected), liver, spleen, peritoneum, lungs, duodenum, pancreas, esophagus, and adrenal glands. 3 Mesenchymal tumors metastasize to similar sites, but more slowly. 5
Treatment Modalities
Complete surgical excision is the treatment of choice for focal gastric tumors and may be necessary in order to obtain tissue for histologic diagnosis. 1,3,12,23,30,31 Cytologic examination of tumor tissue impression smears relates well to histologic diagnosis and may provide useful information for intraoperative planning for the aggressiveness of tumor excision. 27 Segmental resection and anastomosis of the stomach with 1 to 3 cm surgical margins can be curative for dogs with benign antral polyps, adenomas, and leiomyomas. 1,12 Adequate control of large, invasive antral carcinomas requires resection of the pyloric antrum and anastomosis of the pylorus to the stomach while sparing the visible biliary tree (Bilroth I surgery). For some patients, more aggressive resection is required (Bilroth II). 1,3,17 Postoperative supportive care consists of anti-emetics, fluid therapy, possible blood transfusions, and pain control. For dogs and cats with significant resection, a jejunostomy tube placed during laparotomy or total parenteral nutrition (TPN) can provide nutrition for 3 or 4 days after surgery, followed by a low-residue diet.
Surgical excision can also be palliative. 3 Partial gastrectomy decreases bleeding, ulceration, and outflow obstruction. Immediate relief from vomiting for up to 8 months has been reported with partial tumor excision. 3 Neuroglycopenic signs exhibited by dogs with paraneoplastic hypoglycemia resolve within hours of tumor excision. 24,25
Adjuvant therapy is needed for dogs with malignant disease; however, little information is available regarding treatment selection or outcome with chemotherapy. The FAC protocol (5-fluorouracil [5-FU], doxorubicin, cyclophosphamide), gemcitabine, mitoxantrone, and cisplatinum/carboplatinum have been tried with little success. 1,3 There is some risk of gastrointestinal perforation with chemotherapy. Intestinal carcinomas express COX-2, but normal small intestine mucosa does not, suggesting an anti-cancer role for COX-2 inhibitors in the palliative treatment of dogs with gastrointestinal carcinoma. 32 Piroxicam (0.3 mg/kg, PO, SID) may be useful. 33
Recently, many canine gastrointestinal tumors previously identified as LMS were reclassified as GIST, thus originating from gastrointestinal pacemaker cells. 34,35 Application of immunohistochemical stains for smooth muscle actin, desmin, S-100, vimentin and c-kit (CD-117) helps identify these heterogenous mesenchymal cell tumors. 34 Most human and canine GIST, but not LMS, express the c-kit protein, a transmembrane receptor with a tyrosine kinase component believed to be responsible for oncogenesis. 35 In humans, the c-kit–positive GIST is quite resistant to chemotherapy but responds to receptor tyrosine kinase inhibitors (TKIs). 19,36 Orally administered, receptor TKIs have become available for veterinary use and may prove to have a role in the treatment of non-resectable or metastatic c-kit–positive GIST. 19
Prognosis and Survival
Prognosis is highly dependent on tumor type and extent. Postsurgical prognosis for dogs with gastric leiomyoma, adenoma, and hypertrophic polyps is excellent with aggressive excision. 1,2,7,17,31 The few dogs and cats reported with gastric plasmacytomas responded favorably to surgical excision and various combinations of chemotherapy, including doxorubicin, melphalan, prednisone, and cyclophosphamide in dogs, and vincristine, chlorambucil, cyclophosphamide, and prednisone in cats. 8,11 In contrast, most dogs with ACA die or are euthanized for progressive disease and intractable vomiting within 2 to 4 months if untreated. 1,3,17,31 With surgical excision, survival may be extended by months, although there are rare cases of long-term survival. 3,30 Dogs with gastrointestinal LMS that survive the immediate postoperative period have a reported median survival time of about 21 months, even with metastasis. 5 When located in the stomach, GIST may have a better prognosis than LMS. 36
Selected References ∗
U. Bonfanti, W. Bertazzolo, E. Bottero, et al. , Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001-2004) , J Am Vet Med Assoc 229 ( 2006 ) 1130 ;
This paper increases awareness of the utility of impression smears for gastric tumor identification .
M.M. Dennis, N. Bennett, E.G. Ehrhart, Gastric adenocarcinoma and chronic gastritis in two related Persian cats , Vet Pathol 43 ( 2006 ) 358 ;
This paper discusses gastric carcinoma and the potential influences of Helicobacter spp. and Ollulanus tricuspis.
C. London, The role of small molecule inhibitors for veterinary patients , Vet Clin North Am 37 ( 2007 ) 1121 ;
Discusses the application of kinase inhibitors to veterinary patients that have cancer .
K.N. Russell, S.J. Mehler, K.A. Skorupski, et al. , Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990-2003) , J Am Vet Med Assoc 230 ( 2007 ) 1329 ;
This paper distinguishes GIST from tumors previously diagnosed as leiomyosarcoma, which may be clinically relevant .
H.M. Swann, D.E. Holt, Canine gastric adenocarcinoma and leiomyosarcoma: a retrospective study of 21 cases (1986-1999) and literature review , J Am Anim Hosp Assoc 38 ( 2002 ) 157 ;
An excellent review of canine gastric adenocarcinomas and leiomyosarcomas with clinical outcome .
SECTION B Intestinal Tumors
Kerry Rissetto and Kim A. Selting
KEY POINTS
• Intestinal tumors are uncommon in dogs and cats, with lymphoma and adenocarcinoma reported most often.
• Feline intestinal tumors are more commonly found in the small intestine, whereas colorectal tumors predominate in dogs.
• Differentiating lymphoma from lymphocytic-plasmacytic enteritis, although challenging, can be accomplished via full-thickness biopsies, immunohistochemical staining, and polymerase chain reaction (PCR) to detect neoplastic cell antigen receptor rearrangements. With the development of tyrosine kinase inhibitors, gastrointestinal stromal tumors (GIST) expressing c-KIT should be differentiated from smooth muscle tumors to determine if this targeted therapy is an option.
Incidence
Intestinal tumors account for less than 10% of all neoplasms and 20% to 30% of alimentary tumors of dogs and cats in the United States. 1-3 In most reports, lymphoma (LSA) is most common 3-12 and adenocarcinoma (ACA) the second most common intestinal tumor in both species. 13-15 As with many cancers, incidence of intestinal neoplasia increases with age. The mean age at diagnosis for all inttestinal tumors ranges between 10 and 12 years in cats and 6 and 9 years in dogs; for dogs with leiomyosarcoma (LMS) the mean age is 12. 3,8,12,14-25 Young, FeLV-positive cats may develop LSA of any site, including the intestines, although extra-intestinal sites are more common. 8,11,26-31 With the exception of feline large granular LSA, 21 a male predilection is reported for development of intestinal tumors (50%–90%) in both dogs and cats. 15,17,18,20,28,29,32-42 Siamese cats appear to be predisposed to develop intestinal ACA and, perhaps, LSA. 3,8,14,32,43 Male cats and those of Asian ancestry are overrepresented in reports of benign intestinal polyps. 22,44-48 Large breed dogs are more commonly affected with smooth muscle intestinal tumors than are smaller breeds, 39 and collies and German Shepherd dogs may be predisposed to intestinal masses, especially ACA, rectal carcinoma, and polyps. 15,49,50 Mast cell tumors (MCT) are the third most common feline intestinal tumor ( Figure 22-1 ) and, in dogs, have been reported primarily in Maltese, among other miniature breeds. Over 50% of cases in two Japanese reports were Maltese dogs, with males predominating. 51,52
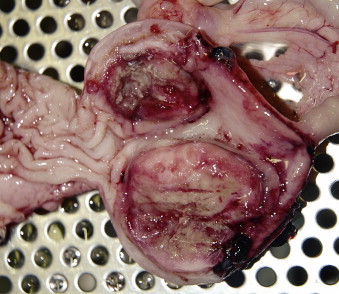 |
| FIGURE 22-1 Mast cell tumors such as the one shown here are the third most common intestinal neoplasm in cats. They also affect small breed dogs, especially Maltese. |
Epithelial, mesenchymal, neuroendocrine, and round cell neoplasia can all occur in the intestinal tract ( Table 22-1 ). The majority of canine intestinal tumors occur in the distal colon, whereas feline intestinal neoplasia is more often reported to occur in the small intestine (SI) primarily in the ileum or jejunum. 2,3,5,6,8,10,15,16,32,34,35,43,53,54 Most SI neoplasia is malignant in dogs, whereas rectal or colonic canine tumors are more likely to be benign polyps, adenomas, or carcinoma in situ 41,55 Recently, evaluation of intestinal smooth muscle tumors has resulted in the reclassification of many of these tumors to gastrointestinal stromal tumors (GIST) ( Table 22-2 ). The GISTs are anatomically more likely to occur in the large intestine (LI), especially cecum ( Figure 22-2 ), compared with the stomach for leiomyomas. 36,56-58 The advent of tyrosine kinase inhibitors (TKIs) as therapeutic agents suggests that differentiation of GIST and LMS is clinically important. Tumors of globule leukocytes originate from leukocytes found between intestinal epithelial cells. They are extremely rare granulated round cell tumors that only occur in cats, commonly in the ileum. 59-62 Few attempts at therapy have been reported, although one cat did well for over a year following surgical resection until the tumor recurred. 62 Isolated case reports mention other rare intestinal tumors including a rectal ganglioneuroma, mesojejunal liposarcoma, and intestinal melanoma. 63-65
| Tumor Type | Species | Location | Characteristics | References |
|---|---|---|---|---|
| Lymphoma (most common overall) | Dog | Stomach = small intestine > large intestine | • Diffuse; infiltration into submucosa and lamina propria • LGL subtype is rare. | 117 |
| Cat | • Subtypes: lymphocytic, lymphoblastic, epitheliotropic (T cell only) and LGL (rapidly fatal) types • T ≥ B cell origin, no association with FeLV status • Unique subtypes usually FeLV negative | 25,33,61,118,119 | ||
| Adenocarcinoma | Dog | Colon and rectum | • Often pedunculated (especially in the distal rectum), cobblestone (middle rectum), or annular (middle rectum) appearance, which may relate to behavior and prognosis. | 13,54,89,95 |
| Cat | Small > large, but in large it is the most common tumor | • Colonic = only intestinal tumor for which sx + doxorubicin chemotherapy yields a survival advantage. | 2,3,5,6,10,12, 34,43,54, 120-123 | |
| Mast cell tumor | Dog | Stomach and small intestine | • Typically poorly granulated • Often positive on IHC for toluidine blue, c-kit, and tryptase • Intestinal mast cells may be structurally distinct from cutaneous | 51 |
| Cat | Distal small intestine and colon | • Third most common intestinal tumor in cats • Similar to, but distinct from, carcinoids • May present as eosinophilic enteritis | 4,124-126 | |
| Leiomyosarcoma (LMS) | Dog (rare in cats) | Cecum | 2,4,5,12,17,43,95 | |
| Gastrointestinal stromal tumor (GIST) | Dog | Cecum | • Ultrastructural appearance and c-kit (CD117) staining can distinguish from LMS. • Arises from interstitial cells of Cajal, which regulate intestinal motility via an autonomic pacemaker effect. | 17,36,56-58,95 |
| Carcinoids | Dog | Large and small intestines | • From the diffuse endocrine system • Histologic similarity to carcinomas • Contain secretory granules that may contain substances such as 5-hydroxytryptamine (serotonin), secretin, somatostatin, and gastrin Immunohistochemistry for cytokeratin and for secretory substances such as serotonin may be positive. • Follow an aggressive and debilitating course and often metastasize to the liver | 3,57,95,127 |
| Extramedullary plasmacytoma | Dog and cat | Most in the oral cavity | • Rare • No systemic signs of multiple myeloma • Slowly progressive and complete excision often gives long-term control. | 128 |
| Extraskeletal osteosarcoma | Cat | Duodenum | Uncommon (3/145 feline OSA) | 129,130 |
| Hemangiosarcoma | Cat | No site predilection | Aggressive, all cats dead within 1 week of surgery (n = 4) | 131,132 |
| Polyps | Dogs | Rectum | Most are solitary, but multiple, diffuse lesions are more likely to recur. | 41 |
| Cats | Duodenum | Solitary, but multiple large masses may be caused by Strongyloides tumefaciens infection | 45-48 |
| GIST , Gastrointestinal stromal tumor; LM , leiomyoma; LMS , leiomyosarcoma. | ||||
| Histologic Malignancy | Stain Positive | Stain Negative | +/- | |
|---|---|---|---|---|
| LM | Benign | • SMA and/or desmin | • S-100 • KIT | • Vimentin |
| LMS | Malignant | • SMA and/or desmin | • S-100 • KIT | • Vimentin |
| GIST | Benign or malignant | • Kit • Vimentin | • | • SMA • Desmin • S-100 |
| GIST-like | Benign or malignant | • Vimentin | • KIT | • SMA • Desmin • S-100 |
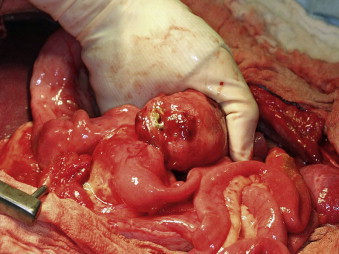 |
| FIGURE 22-2 The cecum is the most common site for gastrointestinal stromal tumors (GISTs), which may become quite large and lead to perforation and peritonitis at the time of presentation. |
Etiology
Molecules involved in anti-adhesion and motility such as tenascin, versican, and hyaluronan have been investigated in canine intestinal tumors and may have differential expression in benign and malignant lesions. 55,66,67 All colorectal adenomas and almost half the carcinomas in one study stained positively for β-catenin, suggesting a role in carcinogenesis similar to that shown in human colorectal adenomatous polyposis coli. 68
Alterations in other molecular processes offer clues as to the pathogenesis of intestinal neoplasia. The tumor suppressor gene p53 has been identified in 15%-50% of canine GI tumors, but does not aid in differentiation of benign from malignant tumors. 68,69 Measures of cellular proliferation such as argyrophilic nucleolar organizer regions (AgNOR) did not correlate with remission rate, duration, or survival time in feline intestinal lymphoma. 24 COX-2 is inconsistently expressed in both benign and malignant small intestinal and colorectal epithelial tumors in dogs, but not in intestinal tumors in cats, 70-72 suggesting a greater value of COX-2 inhibitors in our canine patients.
With the exception of retroviral influence (FeLV and FIV) on the development of feline lymphoma, there are no known etiologic organisms or chemical agents that have been convincingly linked to development of spontaneously occurring intestinal neoplasia in dogs or cats. 14,32 Although previous reports show that older cats with intestinal lymphoma are usually negative for FeLV on serology, one in four of those patients will be PCR tumor positive for FeLV, suggesting a role of FeLV in the pathogenesis of intestinal lymphoma in older seronegative patients. 73 Induction of feline lymphoma in a cat that is serologically negative for FeLV may result from latent, replication-deficient, or partial genome virus infection, or from a “hit and run” incident in which the virus is not incorporated into the host genome. 73 Whereas Helicobacter pylori infection is associated with increased risk of gastric cancer in people, no such association has been confirmed in domestic animals. Concurrent LSA and Helicobacter infection has been reported in a cat, but cause and effect has not been confirmed. 74 Multiple gastroduodenal ACAs and a rectal adenoma were found in a cougar with concurrent Helicobacter -like organisms and spirochetes; whether it was causative or coincidental is unknown. 75 Because some cats shed Helicobacter species in the feces, 76 this may represent normal flora rather than pathogens.
An association exists between cyclosporine use in human transplant patients and the development of LSA. Multicentric lymphoma has been reported in one dog 4 weeks after initiation of cyclosporine and ketoconazole therapy for anal furunculosis, but a causative relationship could not be proven. 77
Clinical and Laboratory Abnormalities
The duration of clinical signs prior to presentation for animals with intestinal tumors averages 6 to 8 weeks, but can range from less than 1 day to several months. 17-19 Clinical signs include weight loss, diarrhea, vomiting, and anorexia, and, less frequently, melena, anemia, and hypoglycemia (with smooth muscle tumors). 3,14,19,28,33,35,37,43 Clinical signs often relate to location of the tumor within the GI tract. Proximal intestinal tract tumors commonly result in vomiting and weight loss, cecal tumors in perforation and peritonitis, and large bowel tumors in hematochezia and tenesmus. 32,40,58 The higher incidence of weight loss associated with SI tumors may result in earlier presentation, smaller size of tumors at the time of diagnosis, and a lower risk of perforation compared with those of the cecum and LI. 58 Russell et al. 57 proposed that the cecal wall is thinner compared with other areas of the intestinal tract and that the tumors commonly found there (GISTs) are more locally invasive compared with other cecal tumors. Because smooth muscle tumors are located within the muscular layer of the intestines, and not within the lumen, evidence of GI bleeding is often absent, but anemia and melena have been reported. 17,20 Anemia is common in dogs and cats with intestinal tumors other than leiomyosarcomas and may occur in conjunction with melena and elevated BUN. 17,19,20,23,32,33,40 Leukogram changes are also common, including leukocytosis in 25% to 70% of dogs and 40% of cats, as well as a left shift and monocytosis. 14,19,20,32,33
Biochemical abnormalities are similar between dogs and cats with intestinal tumors, with malabsorption-related hypoproteinemia affecting 25% to 33% of patients. 14,19,20,23,37,40 Other common abnormalities include elevated liver enzymes, specifically alkaline phosphatase in 15% to 33% of dogs and up to 85% of cats with non-lymphomatous neoplasia. 19,20,32,33,40 Dogs may also have increased amylase and electrolyte disturbances, 40 and patients with lymphoma may be hypercalcemic. 23
Paraneoplastic syndromes are uncommon but can occur with intestinal neoplasia. Neutrophilic leukocytosis has been reported in two dogs with rectal tumors as well as one dog with an intestinal sarcoma, which resolved after treatment. 77-80 Although some cats may present with hyperglycemia, 32 smooth muscle tumors in cats and dogs can cause up to 55% of patients to be hypoglycemic as a result of insulin-like growth factor secretion. 17,81 Hypereosinophilic syndrome has been reported in dogs and one cat with intestinal T cell lymphoma; the suggested cause was interleukin-5 secretion by the neoplastic lymphocytes. 82-84 Extramedullary plasmacytoma may lead to hyperviscosity syndrome resulting from overproduction of immunoglobulin. 85 Additional signs deemed paraneoplastic by their resolution with treatment or identification of an underlying mechanism include erythrocytosis (cecal leiomyosarcoma), nephrogenic diabetes insipidus (leiomyosarcoma), and collapse caused by paroxysmal ventricular tachycardia (neuroendocrine tumor). 86-88
Diagnosis and Staging
Abdominal palpation is a reliable method of identifying an abdominal mass in approximately 20% to 50% of dogs with intestinal tumors 18,19,32,37,40 and 50% to 86% of cats. 14,23,32,33 Other physical findings include pain and fever. 18 Digital rectal examination may identify masses or annular strictures due to rectal tumors or polyps in up to 63% of dogs. 13,40 Dehydration is common in cats with non-lymphomatous tumors. 14,32 In dogs and cats with intestinal lymphoma, concurrent enlargement of liver, spleen, and/or mesenteric lymph nodes may be seen. 18
When intestinal tumors metastasize, sites of predilection in decreasing frequency include mesenteric lymph nodes (especially ACA), liver (especially LMS), mesentery, omentum, spleen, kidney, bone, peritoneum, and lung. 19,32,34,89 Other rare sites of metastasis include testicles, skin, and other visceral organs. 90-92 Lymphoma is often a systemic disease, and 25% of dogs and 80% of cats with gastrointestinal lymphoma have concurrent involvement of other organs. 8,18 Abdominal radiographs reveal a mass in approximately 40% of dogs and cats with intestinal tumors, although some reports are higher for solid tumors and lower for lymphoma because of the diffuse nature of the disease. 14,18-20,23,32 Effusions or visceral organ involvement causing decreased serosal detail may result in decreased radiographic sensitivity. An obstructive pattern may be seen on plain radiographs with incidence ranging from 10% to 75%. 19,20,32,40
Contrast radiography can help visualize an obstruction, localize a tumor, and view areas of the GI tract that are difficult to image with ultrasonography because of gas accumulation. Filling defects may be seen in approximately half of the cats and dogs with GI neoplasia, and most cases will have abnormal contrast series. 14,18,32
Thoracic radiographs are critical to the complete evaluation of the cancer patient; however, for dogs with non-lymphomatous intestinal tumors, pulmonary metastasis is uncommon. 14,17,19,20,32,40,58 For cats and dogs with LSA, enlarged sternal or perihilar lymph nodes, pleural effusion, or diffuse interstitial changes may be seen. 18,23
Ultrasound allows for more sensitive localization of a tumor and evaluation of other abdominal organs for metastasis or involvement. In addition, it can assist with guidance for needle aspiration and needle biopsy. 17,19,39,93 Ultrasound findings that are most consistent with intestinal neoplasia include bowel wall thickening and loss of normal intestinal wall layers. 40,93,94 Intestinal LSA in dogs often results in long segments of thickened bowel, and in cats either a solitary mass or diffusely thickened intestines. In cats with intestinal ACA, asymmetric mixed echogenic masses have been reported, whereas in dogs, hypoechoic masses with an irregular lumen, proximal fluid accumulation, decreased motility, and regional lymphadenopathy have been reported. 35,40,93-95 Smooth muscle tumors are characteristically large (median diameter 4.8 cm) and anechoic to hypoechoic; a muscular layer origin may be evident. Leiomyomas often have a smooth contour. 39
Although not diagnostic alone, ultrasound has proven helpful in differentiating neoplastic from non-neoplastic intestinal disease. Dogs with tumors have significantly thicker intestinal walls, and 99% have a loss of wall layering compared with a maintenance of wall layering in 88% of dogs with non-neoplastic disease. In addition, dogs with walls thicker than 1 cm are nearly 4 times as likely to have a tumor, and those with focal lesions are nearly 20 times as likely. 94 Cats with carcinomatosis typically have masses in the double sheet portion of peritoneum and free peritoneal fluid. 38
Endoscopic findings in dogs with intestinal lymphoma include an irregular cobblestone or patchy erythematous appearance to the duodenal mucosa and poor distensibility and elasticity of the duodenal wall. 37 Endoscopically obtained tissues often facilitate diagnosis; however, partial thickness biopsy samples may limit a diagnosis yield, since transmural neoplastic lymphoid cell infiltration must be identified in order to differentiate GI lymphoma from lymphocytic-plasmacytic enteritis (LPE). 40,53,96,97
When non-invasive or minimally invasive techniques fail to confirm a diagnosis, an exploratory laparotomy may be indicated for a dog or cat with persistent signs of GI disease. Benefits include direct visualization of all abdominal viscera and the ability to collect full-thickness biopsy samples. Surgery may provide both a diagnosis and treatment in those with resectable tumors and can facilitate the diagnosis and evaluation of carcinomatosis. It should be noted that carcinomatosis should not always be seen as a death sentence, since long-term survival has been reported in cases treated with chemotherapy. 14
Immunocytochemistry and IHC for markers such as CK7 may differentiate benign from malignant GI tumors. 98 Likewise, lymphocyte markers and polymerase chain reaction (PCR) can determine clonality of intestinal lymphoma as well as distinguish lymphoma from inflammatory bowel disease. 99,100
Treatment
With the exception of LSA and potentially MCT cases, 51,52 surgical resection is the primary treatment for intestinal tumors and complete excision is often possible ( Figure 22-3 ). 101,102 In the absence of metastasis, long-term survival is possible following surgical resection. The overall 1-year survival rate is approximately 40% for dogs with surgically treated solid (non-lymphoma) SI tumors. 19 Approximately 50% of intestinal ACAs in cats will metastasize to the local lymph nodes, 30% to the peritoneal cavity (carcinomatosis), and up to 20% to the lungs. 3,32,34 Similar lymph node metastasis rates are noted with canine ACA and LMS, although the liver is the second most frequent metastatic site. 19,32,95 Perioperative mortality approaches 30% to 50% as a result of sepsis, peritonitis, or euthanasia for non-resectable intestinal tumors. 17,19
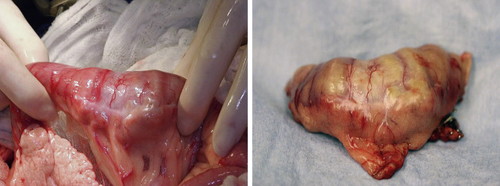 |
| FIGURE 22-3 Surgical resection is the treatment of choice for most intestinal malignancies, such as this duodenal ACA. |
Dogs with colorectal tumors can fare well following surgical removal, with a reported median survival time (MST) of 15 months for extramedullary plasmacytomas, 22 months for colorectal ACA, and 2 years for polyps. 13,49 In contrast, reported survival for dogs with small intestinal ACA is only 12 days without treatment and 4 to 10 months with surgical resection. 19,32,40,103
Dogs with LMS who survive the perioperative period have reported MST ranging from 7.8 to 21.3 months. 17, 20 Those with GISTs also do well, with MST greater than 3 years reported. 57 No significant difference in recurrence free period or overall survival time was found when comparing tumor location (SI or cecum) or tumor type (LMS or GIST). 57,58
Excision of discrete, solitary intestinal lymphoma masses is controversial. Although some clinicians favor excision to prevent a possible post-chemotherapy perforation, others prefer chemotherapy alone with close monitoring of the intestines. When considering intestinal mast cell disease in dogs, the benefit of surgery is questionable. In two case series, the majority of dogs died within 1 month of surgery with only 2 of 49 dogs living longer than 180 days. 51,52
Distal colorectal carcinomas may be excised via pull-through technique. Although short-term complications such as rectal bleeding, tenesmus, stricture, and, uncommonly, chronic fecal incontinence; may occur, long-term survival has been reported. 104,105 In one report bilateral pubic and ischial osteotomy in the surgical management of caudal colorectal masses resulted in no associated complications, and all patients were ambulatory within 3 days of surgery. 106 Because of slower cecal wall healing, staples are indicated in typhlectomies to provide greater resistance against tension, thus minimizing the risk of dehiscence. 107 Colostomy is reported to aid in the management of dogs with non-resectable rectal tumors. In one report, skin excoriation was the most common complication, but colostomy bags were maintained for up to 7 months. 108
Benign canine rectal tumors can be removed endoscopically or surgically, resulting in improvement in quality of life. However, clinical signs recur in 41% of dogs, and transition to malignancy has been reported in 18% of patients. 41,109 Surgical removal of duodenal polyps in cats typically affords a cure. 22
Cats with small intestinal ACA who survive the perioperative period may experience long-term control with surgery alone, with mean survival times ranging from 5 to 15 months. 14,43 Without surgery, all patients in two studies were dead within 2 weeks of diagnosis. 14,32 In cats with LI neoplasia, survival following surgery alone was approximately 3.5 months for lymphoma, 4.5 months for ACA, and 6.5 months for mast cell tumor. Adjuvant chemotherapy improved survival for cats with ACA, but not for cats with lymphoma. 12 Colonic stenting may also be an option for maintaining quality of life in patients with ACA. 110 Following removal of a primary intestinal ACA, two cats with carcinomatosis lived 4.5 and 28 months after surgery. 14 Feline LSA is primarily treated with chemotherapy, with the exception of intestinal obstruction, intestinal perforation, or when the need for a full-thickness biopsy necessitates surgery.
No randomized studies exist to confirm or deny any benefit of adjuvant chemotherapy following resection of intestinal tumors. When attempted, adjuvant chemotherapy for solid intestinal tumors (non-lymphoma) typically includes doxorubicin. Prolonged survivals have been noted in dogs with intestinal LMS and ACA that were treated with chemotherapy after surgery; however, dogs that do not receive chemotherapy may fare equally well. 17,40
Multidrug protocols are used for treatment of lymphoma, but outcome is poor in dogs, with reported survival times of less than 14 weeks. 18,111 Cats do better, with MST typically around 6 to 9 months. 23,28 Surgery and chemotherapy did not improve survival compared with chemotherapy alone for cats with alimentary lymphoma. 25 Cats with small cell, lymphocytic intestinal lymphoma did very well with chlorambucil and prednisone treatment. 35 Large granular lymphoma in cats, however, is an aggressive form of lymphoma with MST of only 57 days with treatment. 112
For carcinomatosis, intracavitary treatment with carboplatin in cats, and cisplatin, carboplatin, mitoxantrone, or 5-flourouracil in dogs may be beneficial. 113,114 Dogs with carcinomatosis, sarcomatosis, or mesothelioma treated with intracavitary carboplatin or mitoxantrone had a significantly improved survival (332 days) compared with those in the untreated group (25 days); pleural and peritoneal effusion had no effect on prognosis. 113
A significant reduction in the size and clinical signs of rectal polyps was noted in eight dogs after receiving piroxicam therapy, either orally or in suppository form, regardless of the presence of tumor-associated inflammation. 71
Radiation Therapy
Radiation therapy is seldom used in the treatment of intestinal tumors for a variety of reasons. In addition to the concern for normal tissue toxicity, radiation is often unnecessary, since local disease control is surgically possible. In a recent retrospective evaluation of complications in dogs undergoing definitive pelvic irradiation for a variety of neoplasms, the authors found an increase in complications in dogs with large radiation fields and those with perineal tumors. 115
Prognosis
Intestinal perforation does not appear to be a negative prognostic factor for LMS, since dogs surviving the perioperative period enjoyed prolonged survival in one series. 17 For colorectal tumors, treatment is prognostic, with local excision significantly better than palliative care. Dogs with annular, obstructing masses survived a mean of 1.6 months, those with nodular or cobblestone masses 12 months, and those with single, pedunculated masses 32 months. 13
For nonlymphomatous SI tumors in dogs, metastasis at the time of surgery results in significantly shorter survival times (3 vs. 15 months) with 1-year survival for dogs with lymph node metastasis only 20% versus 67% without. 19 Patients with visceral metastasis from LMS enjoyed prolonged survival following resection of the primary tumor (over 21 months). 17 In one study, males fared significantly better than female dogs with small intestinal ACA, although the number of females was small. 40
Within a diagnosis of feline intestinal lymphoma, tumor subtype impacts behavior. Cats with lymphocytic small cell lymphoma experienced a 69% complete remission rate with prednisone and chlorambucil for an MST of nearly 2 years, whereas cats with lymphoblastic lymphoma had only an 18% complete remission rate and MST less than 3 months with combination chemotherapy. 35 As with other forms of LSA, the strongest prognostic factor for the intestinal form of the disease in cats is response to treatment. Cats achieving a complete remission indicated by resolution of clinical signs typically fare significantly better than those that do not. 25,33,116 Of 103 cats with lymphoma, 28 of which were intestinal, negative prognostic factors included advanced stage of disease and FeLV status in early stage disease (MST = 3 months if FeLV+ and 17 months if FeLV-) 29 Unlike the case in dogs, immunophenotype does not appear to be a prognostic factor for cats with intestinal lymphoma. 25 If surgery is attempted for feline colonic lymphoma, adjuvant chemotherapy appears to offer no advantage (MST of just over 3 months in both groups). Cats with ACA, however, survive significantly longer if they receive subtotal colectomy (138 days vs. 68 days with mass excision), postoperative doxorubicin (280 days with vs. 56 days without), and have negative lymph nodes at surgery (259 days negative vs. 49 days positive). 12
Selected References ∗
U. Bonfanti, W. Bertazzolo, E. Bottero, et al. , Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001-2004) , J Am Vet Med Assoc 229 ( 2006 ) 1130 – 1133 ;
This report supports the necessity for histopathology to diagnose gastrointestinal tract tumors in our small animal patients .
S.E. Evans, J.J. Bonczynski, J.D. Broussard, et al. , Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats , J Am Vet Med Assoc 229 ( 2006 ) 1447 – 1450 ;
This is a good study determining the efficacy of endoscopic biopsies versus full-thickness biopsies in determining a histologic diagnosis of lymphoma .
C.P. Maas, G. ter Haar, I. van der Gaag, et al. , Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation , Vet Surg 36 ( 2007 ) 302 – 313 ;
This recent review discusses the histologic and immunohistochemical criteria for classifying smooth muscle tumors of the intestinal tract. It also provides a brief review of the two prior studies (Frost and LaRock) discussing similar reclassification criteria .
M.C. Paoloni, D.G. Penninck, A.S. Moore, Ultrasonographic and clinicopathologic findings in 21 dogs with intestinal adenocarcinoma , Vet Radiol Ultrasound 43 ( 2002 ) 562 – 567 ;
This provides a good description not only of ultrasonographic findings of intestinal adenocarcinoma, but signalment, clinical presentation, laboratory findings, treatment, as well as outcome .
N.E. Waly, T.J. Gruffydd-Jones, C.R. Stokes, et al. , Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats , J Comp Pathol 133 ( 2005 ) 253 – 260 ;
This paper reviews immunohistochemical stains as an adjunctive diagnostic tool to differentiate lymphoma from lymphocytic-plasmacytic enteritis in cats, as well as determine immunophenotype .
SECTION C Liver, Gall Bladder, and Non-Endocrine Pancreatic Tumors
Bonnie Brugmann and Mary Lynn Higginbotham
KEY POINTS
• Primary hepatic tumors are uncommon in dogs and cats; metastatic hepatic neoplasia and liver involvement of lymphoma and mast cell disease are more common.
• If resectable, long-term survival with massive hepatocellular carcinoma is common, but nodular and diffuse tumors carry a poor prognosis.
• Exocrine pancreatic tumors are typically aggressive in both dogs and cats, with metastatic disease common at the time of diagnosis.
Incidence—Morbidity and Mortality
Primary hepatobiliary tumors are uncommon in companion animals. They have been reported to comprise between 0.6% and1.3% of all canine neoplasms and between 1.5% and 6.9% of all feline neoplasms. 1-6 Metastatic disease to the liver occurs three times more often than primary neoplasia in the liver of dogs. 3 Other common neoplastic diseases that may involve the liver include lymphoma, mast cell tumors, and histiocytic diseases. The reader is referred to chapters specific to these tumors for detailed information.
Etiology and Risk Factors
The cause of hepatobiliary tumors in companion animals is largely unknown. Chronic diseases of the liver, such as hepatitis B or C infection and cirrhosis, are often associated with hepatocellular tumors in humans 7 ; however, they have not been associated with these tumors in companion animals. 8 A possible association between cholangiocarcinoma and hookworm or whipworm infestation 9 was reported, but this association has not been substantiated. Hepatobiliary tumors tend to occur in geriatric animals, with the majority of animals being 10 years of age or older. 1,10,11
Clinical Features of Canine Hepatobiliary Tumors
Four general categories of primary hepatic tumors exist: hepatocellular tumors, bile duct tumors, neuroendocrine tumors, and sarcomas. Hepatobiliary tumors are also categorized based upon their gross appearance: massive, involving only one liver lobe; nodular, multiple and involving more than one liver lobe; or diffuse, effacement of the hepatic tissue. A summary of the incidence of canine hepatobiliary tumors can be found in Table 22-3 .
| NR , Not reported. | |||||
| Tumor Type | Incidence | Metastatic Rate | Treatment | Survival | Reference |
|---|---|---|---|---|---|
| Hepatocellular carcinoma | 55/110 = 50% | 1 | |||
| Massive | 61% | 4.8%–6.6% | surgery vs. no treatment | >1460 days vs. 270 days | 8, 25 |
| Nodular | 29% | 93% | NR | NR | 8 |
| Diffuse | 10% | 100% | NR | NR | 8 |
| Biliary carcinoma | 24/110 = 22% | 27%–88% | NR | NR | 1, 9, 14 |
| Massive | 50% | NR | NR | NR | 14 |
| Nodular | 28% | NR | NR | NR | 14 |
| Diffuse | 22% | NR | NR | NR | 14 |
| Neuroendocrine tumors | 15/110 = 14% | 93% | NR | NR | 1, 15 |
| Massive | 0% | NR | NR | NR | 15 |
| Nodular | 33% | NR | NR | NR | 15 |
| Diffuse | 66% | NR | NR | NR | 15 |
| Mesenchymal tumors | 14/110 = 13% | 86% | NR | NR | 1 |
| Leiomyosarcoma | 10/14 = 9% | 100% | surgery | euthanized at surgery | 1, 21 |
| Fibrosarcoma | 3/14 = 3% | NR | NR | NR | 1 |
| Osteosarcoma | 1/14 = 1% | NR | NR | NR | 1 |
| Hemangiosarcoma | 6/104 = 6% | NR | NR | NR | 19 |
Hepatocellular Tumors
Hepatocellular adenoma, or hepatoma, is a benign tumor that has infrequently been reported and must be histologically differentiated from nodular hyperplasia and low-grade hepatocellular carcinoma (HCC). 12 Hepatocellular carcinoma is the most common canine liver tumor, 1 and there appears to be a predilection for the left liver lobe with massive HCC. 1,13,14 Multiple histologic types of HCC have been identified but do not aid in determining prognosis. 8 There is an overall reported metastatic rate of 61% for HCC in dogs, most frequently to the lymph nodes, lungs, and peritoneum. 8
Bile Duct Tumors
The majority of feline and canine biliary carcinomas are intrahepatic in origin rather than extrahepatic or of the gall bladder in origin, as they are in people. 9,12,15 Benign intrahepatic biliary tumors, or cholangioma, have been reported in the dog but occur less frequently than the malignant cholangiocarcinoma. Cholangiocarcinomas accounted for 22% of primary hepatic tumors in one study. 16 Biliary tumors in general may be solid or cystic in nature. 12 In dogs, cholangiocarcinomas have a reported metastatic rate of 88%, and the most frequent metastatic sites are the lymph nodes, lungs, and peritoneum. 16
Neuroendocrine Tumors
Neuroendocrine tumors, also termed carcinoids , are of neuroectodermal origin and are rare in domestic species. 1,2,17 They occur throughout the gastrointestinal tract and have been reported in the liver 1,12,17,18 and gall bladder 1,12,17,19 in dogs. In a study of 15 dogs with hepatic carcinoid tumors, a 93% extrahepatic metastatic rate was reported, with the lymph nodes and peritoneum most frequently affected. 17
Sarcomas
Primary liver sarcomas are rare, but predominantly include leiomyosarcoma, hemangiosarcoma (HSA), and fibrosarcoma (FSA). 1,12,20 Primary hepatic chondrosarcoma has been reported in one dog. 21 Although the liver is more commonly a site of metastatic HSA than primary HSA, approximately 5% originate in the liver. 22 No breed predilection has been reported for primary hepatic sarcomas, but males may be at an increased risk. 1 The behavior of these tumors is aggressive with metastatic rates of 57% to 100% reported. 1,22,23 Primary hepatic leiomyosarcomas are uncommon, yet aggressive, tumors. In a report of leiomyosarcomas, 5 of 44 cases occurred in the liver, and all five of those dogs had evidence of metastasis at the time of surgery. 24
Clinical Features of Feline Hepatobiliary Tumors
Hepatocellular, bile duct, neuroendocrine, and mesenchymal tumors have also been reported in cats. Malignant hepatobiliary tumors are more likely to be multifocal (nodular or diffuse) in the cat. 25 A summary of the incidence of feline hepatobiliary tumors can be found in Table 22-4 .
| NR , Not reported. | |||||
| Tumor Type | Incidence | Metastasis | Treatment | Survival | Reference |
|---|---|---|---|---|---|
| Hepatocellular adenoma | 9/41 = 22% | 11 | |||
| 25% | |||||
| 8/47 = 17% | |||||
| 2/25 = 8% | |||||
| Hepatocellular carcinoma | 1/41 = 2% | NR | NR | 10, 22 | |
| Hepatobiliary cystadenoma | NR 5/41 = 12% | 0% | Surgery (n = 5) | 12–44 months | 11, 30 |
| Bile duct | |||||
| Biliary adenoma | 16/47 = 34% 13/25 = 52% 13/41 = 32% | 0% | NR | NR | 10, 11, 22 |
| Biliary adenocarcinoma | 13/47 = 28% 6/25 = 24% 10/41 = 24% | 77% | NR | NR | 10, 11, 22 |
| Gall bladder adenocarcinoma | 2/47 = 4% | 100% | NR | NR | 22 |
| Neuroendocrine | 2/47 = 4% 1/25 = 4% | 100% | NR | NR | 10, 22 |
| Mesenchymal | 6/47 = 13% 3/25 = 12% | NR | NR | 10, 22 | |
| Hemangiosarcoma | 5/47 = 11% 2/41 = 5% | 60% | NR | NR | 11, 22 |
| Leiomyosarcoma | 1/47 = 2% | 100% | NR | NR | 22 |
| Fibrosarcoma | 1/41 = 2% | NR | NR | NR | 11 |
Bile Duct Tumors
Tumors involving the bile duct are more common than hepatocellular tumors in cats and extrahepatic biliary tumors occur more frequently than in the dog. 25 Biliary adenomas are the most common primary hepatic neoplasm in the cat. 10-12,25 A 67% distant metastatic rate has been reported for feline biliary adenocarcinomas. 25
Hepatocellular Tumors
Although hepatocellular adenomas have been reported in cats, hepatocellular carcinomas are more frequent and are the second most common primary hepatic tumor in cats. 10,11,20,25 A distant metastatic rate of 25% has been reported. 25
Myelolipoma
Myelolipoma is a benign primary hepatic tumor that occurs in cats. The etiology is unknown, but they are composed of well-differentiated adipose and myeloid tissues. They may be single or multiple in nature and may involve multiple lobes of the liver. Most cats are asymptomatic for these tumors, since they are typically incidental findings. 12
Diagnosis and Staging
Clinical Findings
Most animals with primary liver tumors present for non-specific signs such as lethargy, weakness, anorexia, vomiting, and polyuria-polydipsia, ∗ yet other animals are asymptomatic. 13 Physical examination reveals hepatomegaly or a cranial abdominal mass in greater than 50% of animals with primary hepatobiliary tumors. 1,3,8,13,26 Less common is the presence of icterus or ascites. 1,10,11 Hepatic encephalopathy and paraneoplastic hypoglycemia have been associated with hepatic tumors and may be a cause for weakness, seizures, or other neurologic signs. 1,3
Blood Work Findings
Common CBC abnormalities for patients with hepatic tumors include a normocytic-normochromic non-regenerative anemia, consistent with chronic disease, and a mature neutrophilia. Thrombocytosis was recently reported to occur in approximately 50% of dogs diagnosed with massive HCC; however, the significance of this is unknown. 14 The biochemical profile typically reflects changes consistent with liver disease. Non-specific liver enzyme elevation is often present with primary hepatobiliary tumors, and in a recent study of canine massive hepatocellular carcinomas, 95% had ALP elevation, 88% had ALT elevation, 66% had AST elevation, and gamma-glutamyl transferase (GGT) was elevated in 54% of dogs. 14 Abnormal clotting profiles have been identified in dogs with hepatic masses; however, the clotting abnormalities are rarely clinically significant. 14,27
Imaging Studies
Radiography and ultrasonography are beneficial in evaluating for the presence of a mass, the extent of involvement, and potential metastatic lesions. A hepatic mass or enlargement typically results in caudolateral displacement of the gastric axis on radiographs. 13,14,26 Peritoneal effusion may result in loss of abdominal detail. Three-view thoracic radiographs should be evaluated for pulmonary metastatic disease or other abnormalities. Abdominal ultrasonography is helpful in identifying hepatic masses; however, a strong correlation between histologic type and ultrasonographic appearance has not been found. 28,29 A recent study using MRI to differentiate benign and malignant focal hepatic lesions found a 94% accuracy rate. 30
Fine-Needle Aspiration and Cytology
Abdominal effusion associated with a hepatic mass may be ascites, or it may be a malignant effusion if peritoneal carcinomatosis is present. A modified transudate is often described, and malignant cells may be present with carcinomatosis. Ultrasound-guided FNA or needle-core biopsy may be helpful in determining the etiology of hepatic masses. Prior to surgical intervention, FNA cytology is recommended if lymphoma or mast cell disease is a strong consideration.
Treatment Modalities
The mainstay of treatment for any primary hepatic mass is surgery. Unfortunately, even with advanced imaging, the resectability of a massive hepatobiliary tumor is difficult to determine preoperatively; thus, exploratory laparotomy is often necessary. 14 The likelihood of complete resection of a nodular or diffuse hepatobiliary tumor is poor, due to the extensive liver involvement. However, because benign hepatic lesions may appear similar to severe, diffuse malignant lesions on gross evaluation, biopsy and histopathological evaluation are warranted prior to final treatment and prognosis discussions with the pet owner. Adjunctive chemotherapy for malignant primary hepatobiliary tumors has not been adequately evaluated.
Prognosis and Survival
Because of their extensive nature and likelihood for metastasis, the prognosis for mesenchymal, neuroendocrine, nodular, or diffuse primary, malignant hepatobiliary tumors in the dog and cat is poor. No reports exist in the veterinary literature evaluating survival of dogs with biliary carcinomas. The outcome for massive hepatocellular carcinomas in dogs, however, appears to be relatively good with surgery, and a recent study of massive HCC in dogs showed a MST of greater than 1460 days in dogs treated with surgery versus 270 days for those not treated. 14 A previous study showed similar results for those that survived the immediate post-operative period. 13 Reported MST for cats with malignant hepatobiliary tumors is 0.1 month. 11 Survival of 12 to 44 months with no recurrence was reported in five cats treated with surgery for benign hepatobiliary tumors. 11,31
EXOCRINE PANCREATIC TUMORS
Incidence—Morbidity and Mortality
Exocrine pancreatic tumors are rare, with a reported incidence of 17.8 and 12.6 per 100,000 patient years at risk in the dog and cat, respectively. 32 They are typically of epithelial origin, and malignant tumors (carcinoma, adenocarcinoma) are more common than benign adenomas. 33 Benign nodular hyperplasia is a common incidental finding in older animals, which can appear as solitary or multi-focal mass-like lesions. 33 Pseudocysts and necrosis have also been described as masses in the pancreas associated with pancreatitis in dogs and cats. 34,35
Etiology and Risk Factors
There is no apparent sex predilection for exocrine pancreatic tumors. 32 Advanced age is a risk factor for exocrine pancreatic carcinomas. 32 Airedale terriers are reportedly at an increased risk. 32 Although there is no known natural cause, experimental intraductal N -ethyl- N ′ -nitro- N -nitrosoguanidine administration has reportedly induced pancreatic adenocarcinoma in dogs. 36 Exocrine pancreatic carcinoma was reported in 8 of 37 cats with diabetes mellitus, 37 which is a risk factor for the development of pancreatic cancer in people. 38 Although suspicious, the relationship has not been proved in cats.
Clinical Features
Clinical signs of exocrine pancreatic tumors in both the dog and cat are non-specific in nature and are often similar to those accompanying pancreatitis. A list of clinical signs and common examination findings can be found in Box 22-1 and Box 22-2 . Paraneoplastic conditions have been reported for dogs and cats with exocrine pancreatic tumors 39-45 and are listed in Box 22-3 . The cause of these conditions is poorly understood; however, panniculitis and steatitis are thought to occur because of hydrolysis of fat by circulating digestive enzymes such as lipase. 43-46 Resolution of paraneoplastic conditions associated with exocrine pancreatic tumors has only been reported in one cat after surgical resection of pancreatic carcinoma 42 and should not be expected, because of the advanced stage of most exocrine pancreatic tumors at the time of diagnosis.
Box 22-1
CLINICAL SIGNS REPORTED WITH PANCREATIC TUMORS IN DOGS AND CATS
• Weight loss
• Vomiting
• Anorexia
• Lethargy
• Palpable abdominal mass
• Abdominal effusion
• Painful abdomen
• Icterus
Box 22-2
COMMON BLOOD WORK FINDINGS ASSOCIATED WITH PANCREATIC TUMORS IN DOGS AND CATS
• Neutrophilia
• Non-specific liver enzyme elevation
• Hyperamylasemia
• Hyperlipasemia
Box 22-3
PARANEOPLASTIC CONDITIONS ASSOCIATED WITH PANCREATIC TUMORS IN DOGS AND CATS
Cats
• Alopecia—symmetrical, ventrum and may extend to the head and medial aspect of extremities 38,39,41
• Panniculitis 44
• Steatitis 44
Dogs
• Panniculitis 42,43
• Steatitis 42,43
• Osteomyelitis 45
• Polyarthritis 45
Diagnosis and Staging
Neutrophilia, liver enzyme elevation, and hyperlipasemia 47 are frequently noted with exocrine pancreatic tumors; however, no specific blood work abnormalities are diagnostic. Radiographic imaging may reveal an abdominal mass or loss of abdominal detail if effusion is present. Ultrasonography is typically of more benefit since it allows for evaluation of the pancreas, liver, and other potential metastatic sites and has been shown to be more sensitive in the detection of pancreatic tumors. 48 Ultrasound imaging may also aid in percutaneous aspiration or biopsy of identified masses for cytologic or histopathologic evaluation. 49 Exploratory celiotomy may be necessary for biopsy procurement for definitive diagnosis. Prior to celiotomy, thoracic radiographs should be performed to evaluate for possible pulmonary metastatic disease.
Metastasis
The biological behavior of pancreatic carcinomas and adenocarcinomas is highly aggressive in both dogs and cats. The presence of metastatic disease is common at the time of diagnosis to the liver and lymph nodes, and widespread metastasis has been reported, 33,45,46,48-51 including intracranial locations. 51
Treatment Modalities
Because of the extensive nature of these tumors and the likelihood that most animals have metastatic disease at the time of diagnosis, treatment is often unrewarding. Complete pancreatectomy or pancreaticoduodenectomy, referred to as Whipple’s procedure, can be considered, but carries significant risks with minimal benefit in light of the aggressive nature of this disease. Before consideration, all efforts should be made to confirm the disease is early stage and localized within the pancreas. Adjuvant therapy is used for human pancreatic carcinoma, but the efficacy of radiation therapy or chemotherapy has not been evaluated for exocrine pancreatic carcinomas in companion animals. 5-FU and gemcitabine are chemotherapeutic agents discussed in people. 52 However, due to fatal neurotoxicity, 5-FU should not be considered for use in cats.
Prognosis and Survival
The prognosis for patients diagnosed with malignant exocrine pancreatic tumors is poor. No long-term reports of survival exist to the authors’ knowledge. A single report exists of a cat with pancreatic carcinoma that survived 18 weeks after resection. Although no evidence of metastasis was found at the time of surgery, metastatic disease was noted on post-mortem examination. 42
Selected References ∗
H.J. Lawrence, H.N. Erb, H.J. Harvey, Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991) , Vet Surg 23 ( 1994 ) 365 ;
Largest clinical study of hepatobiliary masses in cats that discusses diagnosis as well as clinical findings and general survivals .
J.M. Liptak, W.S. Dernell, E. Monnet, et al. , Massive hepatocellular carcinoma in dogs: 48 cases (1992-2002) , J Am Vet Med Assoc 225 ( 2004 ) 1225 ;
Large clinical evaluation of massive hepatocellular carcinomas in the dog. Discusses clinical findings and survival .
A.K. Patnaik, A.I. Hurvitz, P.H. Lieberman, Canine hepatic neoplasms: a clinicopathologic study , Vet Pathol 17 ( 1980 ) 553 ;
Comprehensive case study of hepatic tumors in dogs .
R.L. Seaman, Exocrine pancreatic neoplasia in the cat: a case series , J Am Anim Hosp Assoc 40 ( 2004 ) 238 ;
Clinical evaluation of exocrine pancreatic tumors in the cat. Discusses clinical findings and paraneoplastic conditions associated with this disease in cats .
SECTION D Splenic Tumors
Kim D. Johnson
KEY POINTS
• Dogs with splenomegaly generally follow a law of two thirds: two thirds have splenic neoplasia, and two thirds of those have hemangiosarcoma.
• Dogs presenting with splenomegaly, along with anemia, nucleated red blood cells, abnormal red cell morphology, or splenic rupture have a significantly greater chance of having splenic neoplasia than a non-neoplastic disease.
• Mast cell tumor and lymphoma are the predominate tumors of the feline spleen.
• Splenectomy may provide long-term remission for cats with splenic mast cell tumor.
Introduction and Clinical Behavior
Splenic neoplasia may arise from any of the various tissues comprising the spleen, including blood vessels, lymphoid tissues, smooth muscle, or the connective tissue that makes up the fibrous stroma. Common splenic tumors include hemangiosarcoma (HSA), mast cell tumors (MCTs), lymphoma (LSA), and various sarcomas. 1-3 Hematomas are the most common benign splenic masses in dogs. Of the non-lymphoid primary splenic tumors, HSA predominates in dogs and MCT predominates in cats. 1-3 Splenic LSA primarily occurs as part of multisystemic LSA but may be limited to the spleen in some cases. Feline splenic disease is more likely to be neoplastic, compared with canine splenic disease. 4 In general, dogs with splenomegaly follow a law of two thirds: two thirds have splenic neoplasia, and two thirds of those have HSA. 5
Etiology/Risk Factors
Splenic tumors usually occur in medium to large breed dogs, with the German shepherd dog ranking first in breed prevalence for splenic diseases such as hyperplastic nodule/hematoma, HSA and LSA. Golden retrievers and Labrador retrievers rank second and third, respectively. 4
Clinical Features of Canine Splenic Tumors
Splenomegaly in dogs is readily detectable through abdominal palpation, radiography, and ultrasonography. Differential diagnoses are shown in Box 22-4 . Clinical signs are often vague and vary with the extent of disease. Dogs may be presented for treatment of abdominal enlargement, anorexia, lethargy, depression, diarrhea, or vomiting; alternatively, they may have acute signs of weakness and hypovolemic shock as a result of splenic rupture and hemorrhage. 1,3,6 In a report of 39 dogs with acute non-traumatic hemoabdomen, 24/30 (80%) dogs with definitive diagnosis had malignant neoplasia and 21 of the 24 (88%) malignant masses were diagnosed as HSA. 7
Box 22-4
Get Clinical Tree app for offline access

PATHOLOGIC CAUSES OF SPLENOMEGALY IN THE DOG
Non-neoplastic
Hyperplastic nodule
Hematoma
Splenitis
Abscess
Granulomatous
Congestion
Torsion
Right-sided heart failure
Gastric dilatation and volvulus
Drugs
Infection
Fungal
Bacterial
Viral
Immune-mediated disease
Neoplastic
Benign




Hemangioma
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


