Chapter 18 Treatment of Urinary Disorders
Management of Acute Renal Failure
Pathophysiology
Acute renal failure results from a sudden, severe decline in renal function, which may be initiated by prerenal, renal, or postrenal causes. Although several specific renal diseases can cause acute renal decompensation, in veterinary medicine acute intrinsic renal failure is most commonly caused by a nephrotoxicant or an infectious or ischemic injury. The proportionately high blood flow and large capillary surface area in the kidney increase the organ’s sensitivity to blood-borne toxicants. Additionally, the sophisticated transport mechanisms, intensive metabolic activity, and refined concentrating mechanisms found in renal tubules increase the likelihood of toxic injury. Glomeruli, too, are susceptible to direct destruction and immunologic injury. The most common nephrotoxicants encountered in veterinary medicine are ethylene glycol and therapeutic agents such as aminoglycosides, amphotericin B, cisplatin, radiographic contrast agents, and analgesics. Additional nephrotoxins include lilies, which are toxic in cats only, and raisins and grapes, which are toxic in dogs.1
Ischemic injury may result from any insult that compromises perfusion of afferent arteriolar blood flow. Hypoperfusion from shock, dehydration, or hypotension is the most common mechanism of renal ischemia. Trauma, anesthesia, cardiac output failure, and persistent vomiting or diarrhea are potential ischemic events encountered in small animals. Thrombosis, hyperviscosity, and polycythemia are additional, less common disorders that interfere with renal blood flow. Angiotensin-converting enzyme (ACE) inhibitors, widely used in the management of congestive heart failure in dogs and proteinuric disorders, inhibit production of the vasopressor angiotensin II. In the glomerulus angiotensin II blockade preferentially dilates efferent arterioles, which may lead to loss of glomerular capillary pressure and reduction in glomerular filtration (see Chapter 14). The vasodilatory effect is most prominent in diseased or poorly perfused kidneys and can lead to progressive azotemia or overt acute renal failure in treated patients.2,3
The administration of nonsteroidal antiinflammatory drugs (NSAIDs) may inhibit vasodilatory prostaglandin production in the kidneys. The effect of NSAIDs is minimal in healthy kidneys but can be devastating when superimposed on marginally functioning kidneys, hypovolemia, or other vasoconstrictive states (anesthesia, surgery, sepsis, heart failure, liver failure, nephrotic syndrome).4 In these disorders renal blood flow and glomerular filtration rate become increasingly dependent on prostaglandin synthesis; administration of NSAIDs can precipitate renal ischemia and failure. Many systemic diseases increase the risk of acute renal failure by ischemic or vascular mechanisms. These disorders include pancreatitis, hepatic failure, immune-mediated hemolytic anemia, heat stroke, disseminated intravascular coagulopathy, rickettsial disease, babesiosis, and bacterial endocarditis.5,6
Both toxicant and ischemic insults to nephrons lead to impairment of cellular transport mechanisms, cellular swelling, and death. Cellular hypoxia and intracellular calcium overload lead to additional membrane damage and oxygen free radical formation. Vascular congestion and tubular obstruction result from cellular swelling and act as common mechanisms perpetuating renal ischemia and renal failure.7 Therapeutic measures employed in acute renal failure attempt to support renal excretory function, attenuate cellular damage, and favor renal recovery.
Fluid Therapy
After identification of underlying disorders, the management of acute renal failure relies largely on management of fluid, electrolyte, and acid–base imbalances. Fluid deficits are estimated (estimated percentage dehydration × body weight in kilograms = liters required) and replaced rapidly, within 4 to 6 hours. Initial fluid choices include 0.9% saline or other replacement solutions. Low-sodium fluids such as 0.45% saline/2.5% dextrose or half-strength lactated Ringer’s solution in 2.5% dextrose may be used for patients with cardiac insufficiency or hypernatremia. Fluids for maintenance requirements (40 to 60 mL/kg per day) and ongoing losses (polyuria, vomiting, diarrhea) should be added to the daily fluid total. In most cases rehydration fluid requirements will equal two to three times maintenance requirements; careful calculation of deficits and ongoing needs is recommended to prevent underestimation of fluid needs (Table 18-1).
Urine output should be measured during the rehydration phase to document appropriate diuresis and to calculate future fluid requirements. After adequate volume replacement, urine output should reach at least 1 to 2 mL/kg per hour. Oliguric patients, in which urine output is less than 1 mL/kg per hour, require additional treatment. If the animal is not overhydrated, mild volume expansion may be considered. Administration of an additional 3% to 5% of the animal’s body weight in fluid should eliminate any remaining, undetected volume deficits and enhance renal perfusion and glomerular filtration rate (GFR).8 If volume expansion is attempted, the patient must be carefully monitored for signs of overhydration, including inappropriate weight gain, systemic hypertension, increased bronchovesicular sounds, tachypnea, tachycardia, restlessness, chemosis, and serous nasal discharge. Note that dry mucous membranes can be a consequence of uremia and are not good indicators of hydration status in acute renal failure.9 Appropriate volume expansion is documented by a modest increase in body weight and modest reductions in the hematocrit and plasma protein concentrations. Volume overload is a common complication of fluid therapy in oligoanuric renal failure patients. Roughly two thirds of dogs and cats referred for hemodialysis management of uremic crises are hypervolemic. Without dialytic support this complication can be difficult to reverse unless urine production increases.9
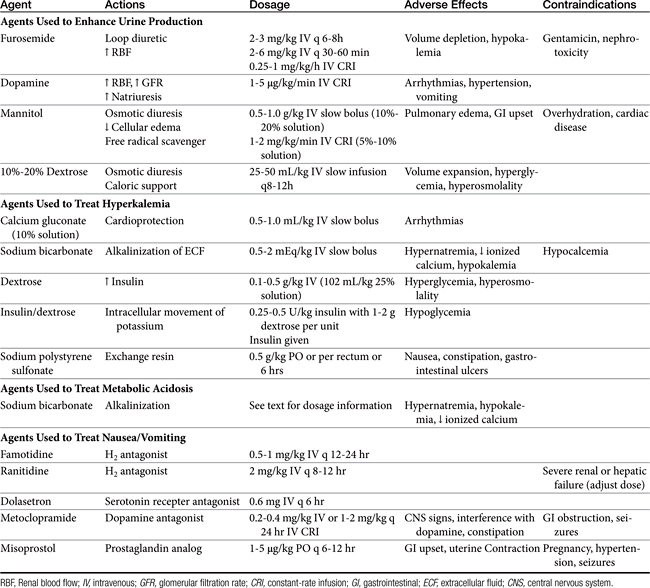
Methods to Enhance Urine Production
If urine production remains poor after rehydration and volume expansion, pharmacologic manipulation of oliguria is warranted. Furosemide, dopamine, and osmotic diuretics have been standard options for management of oliguric and anuric renal failure despite a lack of clinical studies confirming efficacy (see Chapter 17 and for dopamine, see Chapter 14). Despite the lack of proven clinical efficacy, furosemide (2 to 3 mg/kg intravenously every 6 to 8 hours) is often chosen as an initial treatment for oliguria because it is readily available and easy to administer. A constant-rate infusion (CRI) of furosemide (1 mg/kg per hour) has also been recommended.8,10
As a loop diuretic, furosemide helps increase tubular flow and improve renal blood flow but does not significantly affect GFR.7 It is also speculated that the activity of furosemide may protect cells of the thick ascending loop of Henle by reducing active transport at this site. Furosemide may be useful in managing overhydration and hyperkalemia and enhancing toxin elimination in acute renal failure.9 Furosemide has been shown to exacerbate gentamicin toxicity and should be avoided in patients recently treated with aminoglycosides.11 If urine output does not increase in 30 to 60 minutes, furosemide may be repeated at 4 to 6 mg/kg intravenously at 30- to 60-minute intervals; concurrent dopamine administration should also be considered. The efficacy of furosemide in reversing oliguria appears to be improved with the concurrent administration of dopamine.12 Dopamine in this instance may improve delivery of furosemide to sites of activity. If diuresis is established with furosemide administration, intravenous bolus doses may be repeated every 6-8 hours or a CRI can be maintained at 0.25 to 1 mg/kg/hr.9 In healthy Greyhounds a furosemide infusion resulted in more diuresis, natriuresis, and calciuresis and less kaliuresis than bolus doses.13 High-dose furosemide did not increase survival rates in people with acute renal failure in a prospective, double-blinded, randomized placebo-controlled trial.14 An influence on survival in cats and dogs has not been established. Extracellular fluid volume and potassium requirements should be carefully addressed during furosemide treatment.
Dopamine is a catecholamine (a norepinephrine precursor) that in low doses causes increases in renal blood flow. In dogs dopamine acts at specific splanchnic and renal receptors to cause efferent arteriolar vasodilation, enhancing renal blood flow and sodium excretion. Dilation of mesenteric, coronary, and intracerebral vascular beds also is expected. Effects on GFR are modest.15 In cats dopamine appears to stimulate α-adrenergic receptors, leading to increased blood pressure and natriuresis.16 Dopamine must be administered as a CRI, ideally with an automated fluid infusion pump. Dopamine is administered diluted in nonalkaline fluids, usually normal saline or dextrose solutions. Infusion rates of 1 to 5 μg/kg per minute are recommended. Infusion is usually started at 1 to 2 μg/kg per minute while the patient is monitored for changes in heart rate or rhythm. Tachycardia, ectopic or premature ventricular beats, nausea, vomiting, and hypertension are adverse effects, predominantly seen at higher doses. The pressor effects of dopamine are variable and can be detrimental to renal function; monitoring of urine output and degree of azotemia is imperative in individual patients. The half-life of dopamine is approximately 2 minutes; effects are withdrawn within 10 minutes after the infusion is discontinued. The drug is metabolized to inactive compounds by monoamine oxidase and catechol-O-methyltransferase in the kidney, liver, and plasma.17 Recent prospective, randomized, double-blinded, placebo-controlled trials in humans have failed to show efficacy of low-dose dopamine in management of acute renal failure. Dopamine may also cause detrimental gastrointestinal, respiratory, endocrine, and immunologic effects.18 Use of dopamine for management of acute renal failure has fallen out of favor, except as needed for pressor response.
Fenoldopam and other new selective dopamine subtype DA-1 receptor agonists may more effectively increase renal blood flow in dogs8 and possibly in cats. Results of these selective dopaminergic compounds have not been reported in clinically affected patients in acute renal failure.
Osmotic diuretics currently represent the optimal pharmacologic option for enhancing urine flow. Osmotic agents such as mannitol enhance urine production by increasing both intravascular volume and tubular fluid flow. Mannitol is freely filtered at the glomerulus and poorly reabsorbed in renal tubules, creating an osmotic effect such that water is not reabsorbed from the tubular lumen. Osmotic agents also prevent tubular and vascular obstruction by minimizing cellular swelling. Mannitol also possesses weak renal vasodilatory and cellular free radical scavenging actions.19 Adverse effects of mannitol infusion include volume overload and pulmonary edema, gastrointestinal upset, and central nervous system effects (usually at high doses). The drug is contraindicated for overhydrated or dehydrated patients and for patients with preexisting cardiac disease or suspected intracranial hemorrhage. Mannitol (20% to 25% solution) may be administered at a dosage of 0.5 to 1.0 g/kg intravenously as a slow bolus (over 15 to 20 minutes).20 Another protocol entails administration of partial dosages (0.5 mg/kg each) every 15 minutes for three treatments.21 Urine output should improve within 1 hour. A second bolus may be attempted if the agent is unsuccessful, but the potential for volume overexpansion and edema formation increases. When mannitol is beneficial, intermittent bolus injections (0.5 to 1 g/kg intravenously every 6 to 8 hours) or CRI of a 5% to 10% solution (2 to 5 mL/min) may be given up to 2 g/kg per day.13 Lower doses given more frequently (0.25 to 0.5 g/kg intravenously every 4 hours) or a CRI of 1 to 2 mg/kg/min also have been recommended.9 One author recommends maintaining diuresis with an infusion of mannitol diluted in lactated Ringer’s solution.8
Hypertonic dextrose solutions have been useful as an alternative osmotic agent. Once the renal threshold for glucose transport has been exceeded, dextrose solutions create effects similar to those of mannitol on tubular flow and urine output. Solutions of 10% or 20% dextrose are formulated and administered as intermittent slow boluses of 25 to 50 mL/kg (over 1 to 2 hours) two or three times per day. The initial infusion rate may be as high as 2 to 10 mL/min in order to rapidly create hyperglycemia. The infusion rate may subsequently be dropped to 1 to 5 mL/min.21,22 Advantages of dextrose solutions include low cost, availability, and relative safety. Dextrose solutions also provide nominal caloric supplementation. Urine glucose is easily monitored to ensure that sufficient hyperglycemia and filtration of glucose are continuing; urine volume still must be quantitated because glycosuria can occur without significant increases in urine production. Dextrose solutions may be inferior to mannitol in other respects, however, because the osmotic effects on cellular swelling and tubular obstruction will be minimized by intracellular equilibration of glucose across cell membranes, an effect that does not occur with mannitol.8 Hypertonic glucose also lacks the vasodilatory and free radical scavenging effects of mannitol. The rapid movement of glucose intracellularly does, however, minimize the potential development of vascular overload and pulmonary edema.
For patients who become fluid overloaded and have decreased renal output, fluid removal is an essential part of management. Standard treatment in human medicine that is also available for veterinary patients includes peritoneal dialysis and hemodialysis. A promising new treatment is cross-linked polyelectrolyte sorbents. This oral solution can absorb as much as 50 times its weight in gastrointestinal water (up to a liter of fluid in a 30 kg dog) as well as urea, creatinine, and potassium, allowing an alternative to renal excretion of excessive fluid and solutes.23
The choice of initial treatment protocol for oliguria varies with clinician preference, experience, available technical support, and patient variables. Furosemide or dopamine (or both) historically were employed initially, but this practice must be critically assessed because dopamine may actually cause detrimental effects. Mannitol is probably, however, the preferred agent for treatment of nephrotoxic and ischemic renal failure in patients that are not overhydrated. If one protocol is ineffective, another protocol may be attempted. Polyuric renal failure generally is easier to manage and has a better prognosis than oliguric renal failure. The effects of all measures to reverse oliguria appear to diminish as the duration of oliguria is prolonged.
Management of Hyperkalemia and Metabolic Acidosis
Patients with acute renal failure may be hypokalemic, normokalemic, or hyperkalemic. Hyperkalemia is most likely observed with oliguric or anuric renal failure. Management of hyperkalemia and other electrolyte disturbances is ideally based on serum electrolyte determinations; however, an estimate of potassium status can often be made on the basis of an electrocardiogram. Administration of potassium-free fluids and initiation of a diuresis is usually sufficient to correct mild to moderate hyperkalemia. Longer-term control of mild hyperkalemia may be gained with exchange resins. Sodium polystyrene sulfonate (Kayexalate) is given as a suspension in 20% sorbitol (2 g/kg/day by mouth or by rectum in 3 to 4 divided doses). Nausea, constipation, and gastrointestinal ulceration or erosion are possible complications of this product and have limited its tolerance in veterinary patients.9
Peaked T waves, bradycardia, prolonged PR intervals, flattened P waves, and widened QRS complexes may be seen with moderate elevations in serum potassium. Severe hyperkalemia may result in a loss of P waves, idioventricular rhythms, atrial standstill, or ventricular fibrillation and represents a life-threatening emergency. With severe electrocardiographic changes, administration of calcium gluconate (0.5 to 1 mL/kg of a 10% solution given intravenously over 10 to 15 minutes) offers cardioprotective actions. Calcium ions counteract potassium without lowering serum potassium; other measures must be initiated to prevent subsequent cardiac toxicity.24
Bicarbonate administration facilitates an intracellular shift of potassium ions and is another useful initial treatment for moderate hyperkalemia. Sodium bicarbonate is administered as a slow intravenous injection of 0.5 to 2 mEq/kg.24 Alternatively, bicarbonate deficits can be determined on the basis of serum bicarbonate, total CO2, or base deficit measurement. The deficit is calculated by the formula: 0.3 × body weight (kg) × base deficit or (20 − serum bicarbonate or total CO2 concentration). A portion of the deficit (usually one fourth or one half) is given as a slow bolus or in fluids, and the acid–base status is reassessed. An advantage of sodium bicarbonate administration is concurrent correction of coexisting metabolic acidosis. In the absence of hyperkalemia, bicarbonate administration is reserved for severe acidosis (blood pH <7.2 or total CO2 <12 to 15 mEq/L). Overzealous bicarbonate administration may have serious detrimental results, including hypernatremia, hyperosmolality, ionized calcium deficits, reduced plasma potassium concentrations, metabolic alkalosis, and paradoxical acidosis of the cerebrospinal fluid.
An alternative method of therapy for acute hyperkalemia includes the administration of glucose (dextrose 0.1 to 0.5 g/kg as a 20% solution or 1 to 2 mL/kg 50% dextrose diluted to 25%).20 Administration of glucose triggers endogenous insulin secretion; both glucose and insulin facilitate intracellular movement of potassium. Protocols utilizing insulin and glucose (0.25 to 0.5 U/kg insulin followed by 1 to 2 g glucose per unit of insulin administered) have also been recommended. Exogenous insulin administration can promote hypoglycemia; blood glucose monitoring is required.
Maintenance Fluid Therapy
Fluid composition during maintenance therapy should be tailored to the individual. Polyionic replacement solutions that provide buffering activity and electrolyte replacement (e.g., lactated Ringer’s solution, Normosol-R, Plasma-Lyte 56) may be administered during the first few days of treatment, especially if gastrointestinal or electrolyte losses are great. For longer-term therapy, lower sodium solutions designed to meet maintenance fluid needs (e.g., half-strength lactated Ringer’s solution or 0.45% saline in 2.5% dextrose, Normosol-M, or Plasma-Lyte 56) are preferred, insofar as most ongoing losses will consist of free water losses in polyuria.8 Alternating administration of 5% dextrose solutions with high-sodium replacement solutions may also be effective in preventing hypernatremia in patients requiring long-term fluid therapy.21 Potassium supplementation in excess of amounts supplied in commercial fluids is usually required during the maintenance phase of treatment; a total of 20 to 30 mEq KCl per liter of fluid administered is typically sufficient.
Other Considerations
Multiple complications may be encountered during the course of treatment of acute renal failure. Complications are usually a result of uremia and include oral ulceration, vomiting, diarrhea, malnutrition, infection, hemorrhage, anemia, hypertension, and neurologic deterioration. Most of these complications are best ameliorated by minimizing azotemia. Anorexia and vomiting are typically due to activation of the chemoreceptor trigger zone, uremic gastritis, and mucosal intestinal ulceration. Management of gastrointestinal complications of uremia is described in the later discussion of chronic renal failure. Note that metoclopramide and histamine-2 receptor blockers such as ranitidine and famotidine are renally excreted; doses should be modified for severely uremic animals. Aggressive nutritional support may be required in patients with acute renal failure undergoing long periods of treatment. A diet providing 2 to 3 g protein/kg per day and 70 to 110 kcal/kg per day is optimal for critically ill patients with renal failure. A reduced protein diet is designed to minimize uremia and acidosis associated with acute renal failure. In recovering, mildly azotemic patients, a high-protein diet may enhance renal recovery.25
Cellular Protectants
Many agents have been investigated as potential cellular protectants or stimulants of cellular regeneration in acute renal failure. Agents such as magnesium, adenosine triphosphate (ATP), thyroxine, and glycine have been considered for their potential to restore intracellular energy stores. Atrial natriuetic peptide (ANP), brain natriuetic peptide (BNP), and other similar peptides increase GFR and have renoprotective effects in ischemic renal injury. ANP has caused severe hypotension in patients with renal failure, but BNP appears to increase GFR without causing systemic hypotension.26 Oxygen free radical scavengers and calcium channel blockers have been investigated as methods of alleviating reperfusion injury in renal epithelial cells. Growth factors may promote cellular repair and regeneration. Most of these agents remain in the experimental stages, however, and have found limited clinical application in human or veterinary medicine. Manipulation of the cell biology of acute renal failure is likely to provide therapeutic options in the future, however.27,28
Management of Chronic Kidney Disease
Many varied insults can lead to progressive renal dysfunction in small animals. Infectious diseases, obstructive disorders, hypercalcemia, glomerular disease, and some neoplastic disorders may be identified and specific treatment pursued. In many cases, however, a specific etiology is not determined, and management is directed toward alleviation of clinical signs; correction of metabolic consequences; and, ideally, slowed progression of the disease process. Principles of medical management are to (1) stage the disease and pursue appropriate diagnostic strategies, (2) consider renoprotective maneuvers that may retard progression of renal damage, (3) identify and manage sequelae of renal failure (hypertension, anemia, metabolic acidosis, and gastrointestinal ulceration), (4) intervene as necessary in crises, and (5) plan and initiate appropriate monitoring and follow-up evaluations. Excellent reviews of staging criteria and management considerations are available,29 and updated guidelines are available from the International Renal Interest Society (IRIS) at iris-kidney.org. In general, dietary and other therapeutic maneuvers should be instituted in a stepwise approach, with serial monitoring implemented to tailor management (Figure 18-1).
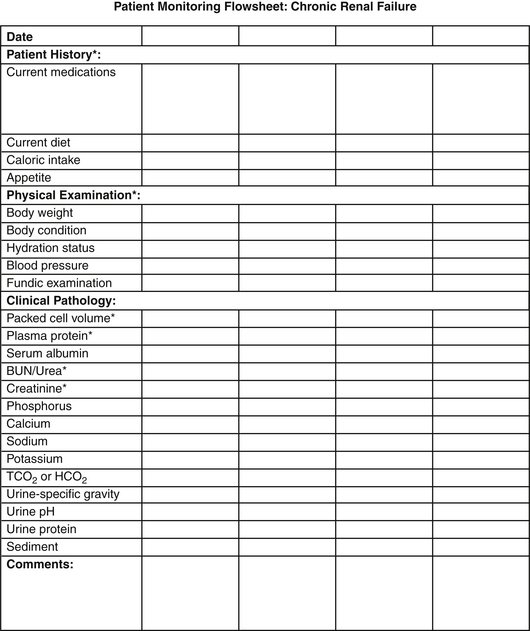
Figure 18-1 Example of a flowsheet for monitoring the clinical and clinicopathologic features of chronic renal failure.
Pathophysiology
The clinical and pathophysiologic consequences of renal disease result from complex events set into motion as excretory, homeostatic, and other renal functions are lost. When approximately 66% of total nephron mass is lost, fluid excretion per nephron is increased to facilitate waste excretion. Solute diuresis in remaining nephrons and developing tubular dysfunction lead to polyuria and compensatory polydipsia. As nephron loss progresses to 75% or greater, excretory function is compromised and azotemia develops. With progressive reduction in GFR, excretion of phosphorus and endogenous acids is impaired, leading to hyperphosphatemia, hypocalcemia, metabolic acidosis, and secondary hyperparathyroidism. Diseased kidneys also fail to produce or regulate other important metabolic and endocrine compounds, leading to systemic hypertension, anemia, and a catabolic state. Widespread polysystemic effects of uremia are possible as well, affecting gastrointestinal mucosa, neuromuscular function, cardiopulmonary function, and immunologic function. Management strategies are designed to blunt these effects of progressive renal dysfunction (Table 18-2).
Dietary Strategies
Protein
Reduction in protein intake (compared with protein content of maintenance commercial dog foods) has been advocated for dogs and cats with renal disease. Dietary protein restriction has been advocated on the basis of the hyperfiltration theory of progressive renal disease.30 In rats with induced renal disease, the compensatory response of remaining nephrons includes increases in single nephron blood flow, single nephron filtration rate, and elevated glomerular capillary pressure.31 These responses are ultimately detrimental in rodent models, leading to progressive renal injury,30 an effect that can be blunted by reduced protein diets that minimize glomerular hypertension.
Although glomerular hypertension and hypertrophy occur in dogs with experimental renal disease,32 a significant effect of protein restriction alone on the course of renal failure in dogs or cats has not yet been demonstrated.33–36 Reduction in protein intake is undeniably beneficial in moderately to severely affected patients by reducing the production of nitrogenous wastes and acid by-products that contribute to uremia and metabolic acidosis. In such patients (usually with blood urea nitrogen >60 to 75 mg/dL or mmol/L), moderate restriction of protein intake can be expected to reduce blood urea concentrations, alleviate metabolic acidosis, and indirectly minimize phosphorus intake.37 Recommended dietary protein intake for initial management is 2 to 3.5g/kg per day in dogs. This level is generally provided by diets containing high biologic value protein at approximately 13% of gross energy when fed at maintenance caloric requirements.37 The protein requirement for dogs in renal failure is higher than the minimum protein requirements for healthy dogs; however, most commercial maintenance diets are 20% to 30% protein. In cats protein requirements are 3.5 to 4.0 g/kcal per day and may be provided by diets containing approximately 21% of gross energy as protein.37 Products that provide high-quality protein in homemade diets include eggs, liver, cottage cheese, and lean meats.
Further reduction in protein intake should be reserved for refractory patients in which signs of uremia persist on the previously described diet. Excessive protein restriction may lead to protein malnutrition, hypoalbuminemia, and anemia. Protein or other nutrient deficiencies can inadvertently develop if adequate quantities of a moderately restricted diet are not consumed; intake of adequate energy should take precedence in dietary formulations. Protein depletion also may adversely affect renal function by contributing to alterations in renal hemodynamics and accentuating muscle catabolism, anemia, and acidosis. Animals with protein malnutrition exhibit weight loss, poor hair coats, and muscle wasting. Although reduced protein intake may improve clinical signs of renal disease, this dietary maneuver is unlikely to prevent renal disease in normal animals, dramatically slow progression of renal disease, or enhance renal function. Thus the role of reduced protein intake in animals with early renal disease is less clear. Again, moderate restriction of protein may be appropriate in these individuals, with regular monitoring for evidence of protein malnutrition and for progression of azotemia.
Phosphorus
Restriction of dietary phosphorus is advocated for patients with renal failure to minimize hyperphosphatemia, secondary hyperparathyroidism, and dystrophic mineralization. Feeding to maintain a calcium × phosphorus solubility product below 60 to 70 is recommended to minimize soft tissue and renal mineralization. In experimental studies in dogs with induced renal disease, phosphorus and calcium restriction improved survival times but did not prevent renal mineralization.38 In a prospective clinical study in cats, feeding a phosphorus- and protein-restricted diet, and administering phosphorus binders if needed, improved survival times from a mean of 383 days to 616 days.39 Cats fed the renal diet also had reduced plasma urea and phosphorus concentrations when compared with cats fed other diets.
Although phosphorus restriction can be advocated more reliably and earlier than protein restriction, most diets formulated for renal disease are restricted in both protein and phosphorus content because meat proteins are the primary source of phosphorus in the diet. Appropriate canine diets are 0.13% to 0.28% phosphorus on a dry weight basis, providing 0.3 to 0.5 mg phosphorus/kcal, whereas feline diets are approximately 0.5% phosphorus, providing 0.9 mg phosphorus/kcal.40 Supplemental phosphate-binding agents may be required if dietary restriction is inadequate to minimize hyperphosphatemia and normalize the calcium × phosphorus solubility product (see later discussions of dietary supplements and secondary hyperparathyroidism).
Sodium
Moderate sodium restriction is beneficial for dogs with renal disease, particularly those with systemic hypertension. Although single nephron adaptive responses are remarkably efficient for maintaining solute and water balance in renal disease, handling of large fluid and solute loads is limited, and conservation of water and solute is impaired. Sodium excretion increases with declining GFRs to maintain homeostasis; however, response to a sodium challenge may be impaired, and excess sodium intake could lead to volume expansion. Conversely, sodium cannot be maximally conserved in the presence of acute restriction in intake or volume depletion. Diets should provide 15 to 50 mg/kg per day, usually 0.1% to 0.3% on a dry matter basis.41 Changes in sodium intake should be made gradually if possible to prevent rapid changes in fluid homeostasis and extracellular fluid volume. A recent study of the effects of dietary sodium intake on renal function in normal cats and cats with experimentally induced renal disease indicated that low sodium intake may actually contribute to hypokalemic nephropathy and progressive renal injury in cats.42 More research must be done to determine the optimal level of sodium intake for cats with renal insufficiency or failure.
Lipids
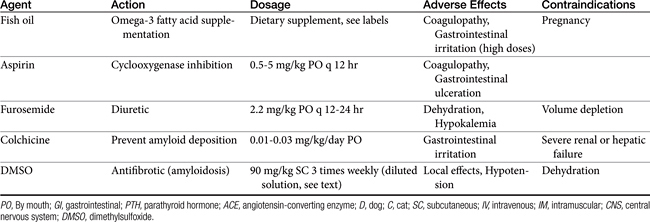
Abnormalities of lipid metabolism in renal disease may lead to hypercholesterolemia, hypertriglyceridemia, and elevated low-density lipoprotein concentrations. High saturated fatty acid intake has been shown to accelerate glomerulosclerosis and progressive renal injury in rat models. Dietary lipid composition may be manipulated to minimize hypercholesterolemia, minimize inflammation, and protect renal hemodynamic function. In one study of dogs with induced renal failure, dogs fed a diet supplemented with omega-3 polyunsaturated fatty acids (provided by menhaden fish oil) had reduced intraglomerular pressure, reduced proteinuria, and better indices of renal function than dogs supplemented with omega-6 polyunsaturated fatty acids.43 Supplementation of omega-3 polyunsaturated fatty acids may be expected to favor vasodilatory eicosanoid production, inhibit intrarenal platelet aggregation, and minimize systemic and glomerular hypertension. Whether any appreciable effect of dietary lipid manipulation will be seen over the long-term course of chronic renal failure remains unknown.43,44
Energy
Appropriate caloric intake is a frequently overlooked goal of dietary management of renal failure patients. A catabolic state may be perpetuated despite the best manipulations of dietary content if sufficient calories for body energy requirements are not ingested. Energy depletion and protein malnutrition in turn exacerbate azotemia and hamper renal compensatory or regenerative responses. Energy requirements for patients with chronic renal failure have been estimated at 60 to 110 kcal/kg per day; a reasonable starting point is 75 kcal/kg per day.31 Frequent monitoring of body weight and body condition is imperative to ensure that appropriate weight is maintained.
Dietary Supplements
Intestinal Phosphate-Binding Agents
If dietary phosphorus reduction is ineffective in maintaining a serum phosphorus level of less than 6 mg/dL and a calcium × phosphorus solubility product less than 70, phosphorous-binding agents may be administered.45 These agents are generally ineffective if dietary phosphorus is not restricted concurrently and are most effective when given just before or with a meal. Liquid or encapsulated preparations are preferable to tablet forms because they more readily mix with ingesta in the intestinal tract. These agents prevent absorption of ingested phophorus or phosphorus secreted in saliva, bile, or intestinal fluid. Tablet forms can be crushed and given with food. Aluminum-based products (aluminum hydroxide, aluminum carbonate) are widely available and are administered at daily dosages of 30 to 100 mg/kg divided into two or three feedings.46 Magnesium-based products should be avoided in patients with renal failure.45
Calcium-based products (calcium acetate, 60 to 90 mg/kg per day; calcium carbonate, 90 to 150 mg/kg per day) are alternative phosphorus-binding agents with additional alkalinizing effects. Calcium-based products can also be used to minimize or correct hypocalcemia. Calcium acetate is recommended in normocalcemic to mildly hypercalcemic patients, insofar as calcium carbonate is more likely to lead to hypercalcemia. Calcium-based products and aluminum-based agents also may be administered concurrently for added phosphorus-binding effects.37,45 Serum calcium and phosphorus concentrations should be monitored every 2 weeks initially, then monthly or as needed during chronic therapy. Adverse effects of phosphorus-binding agents include nausea, gastrointestinal upset, constipation, and hypophosphatemia. Toxic effects of aluminum are theoretically possible with long-term administration, including anemia, encephalopathy, and osteomalacia.47 Aluminum toxicity appears to be unlikely in dogs and cats.
Alkalinizers
Although dietary protein restriction helps reduce acid metabolites and metabolic acidosis, alkalinization therapy may be required in animals with moderate to severe metabolic acidosis. Chronic untreated metabolic acidosis may accelerate protein catabolism and azotemia, promote renal ammoniagenesis contributing to progressive renal tissue damage, and lead to increased calcium and potassium losses. Acid–base derangements also likely contribute to the clinical manifestations of renal failure, including anorexia, vomiting, and weight loss.48,49 Alkalinizing therapy is ideally planned on the basis of serial blood gas analyses. Serum total CO2 (Tco2) measurement is a reasonable guide to management in most patients. Oral alkalinization is recommended when bicarbonate or Tco2 measurements fall below 15 to 17 mmol/L, whereas parenteral supplementation may be needed if the Tco2 falls below 10 to 12 mmol/L.
Alkalinizing agents include potassium citrate (35 mg/kg orally every 8 hours or 0.3 to 0.5 mEq potassium/kg orally every 12 hours) and calcium carbonate or calcium acetate (100 mg/kg daily).31,50 These agents are particularly valuable when hypokalemia (potassium citrate), hyperphosphatemia, or hypocalcemia (calcium-based agents) is a concurrent problem (discussed elsewhere). Oral sodium bicarbonate may be administered at 8 to 12 mg/kg every 8 to 12 hours (1 mEq/cat every 8 to 12 hours for cats). Household baking soda supplies approximately 4000 mg bicarbonate per teaspoon (or 12 mEq bicarbonate/g); 5- and 10-grain tablet preparations are also available. Alternatively, a 1 mEq/mL solution of bicarbonate can be prepared by adding 5 or 6 tablespoons of baking soda to 1 L of water (or one third of an 8 ounce box is added to 1 quart of water).48 Because of the added sodium intake, sodium bicarbonate may be inadvisable in hypertensive patients, and some clinicians prefer to use alternative alkalinizing agents in all renal failure patients. It is questionable, however, whether the sodium salt in sodium bicarbonate contributes to hypertensive disease in dogs and cats.
Potassium
Renal failure and metabolic acidosis have been identified as risk factors for hypokalemic myopathy in cats.51,52 Increased fractional excretion of potassium is observed, although 24-hour potassium loss is variable. Hypokalemia may be exacerbated by chronic metabolic acidosis, especially in cats fed acidifying diets. Potassium depletion in turn induces acidosis and depresses GFR, intensifying renal disease and potassium loss.52–54 From these observations it has been hypothesized that supplementation of potassium in cats with renal insufficiency may stabilize or improve renal function. A beneficial effect on renal function and overall outcome in chronic kidney disease has not yet been proven; however, potassium supplementation can increase total body potassium somewhat and appears to be well tolerated.
Most commercial renal diets have been adjusted to provide potassium beyond requirements for healthy animals, but cats with mild to moderate hypokalemia (K 3.5 to 4.5 mEq/L) will benefit from potassium supplementation at 2 to 5 mEq/day. Low-dose supplementation (2 mEq/cat per day) may be justified in normokalemic cats to prevent potassium depletion.52 Cats with severe hypokalemia may require intensive replacement with intravenous potassium chloride or increased supplementation (5 to 10 mEq/day). Potassium supplementation may be initiated at 1 to 6 mEq/kg per day if other dietary measures do not correct the hypokalemia.31 Potassium concentration should be carefully monitored in cats and in dogs undergoing fluid diuresis for renal disease. Chronically, dogs with polyuric renal failure also may become hypokalemic but seem more resistant to this sequela of renal disease. Some renal failure diets may in fact oversupply potassium for canine needs.
Summary of Dietary Recommendations
On the basis of current information, the ideal diet for small animals in mild to moderate chronic renal failure should be moderately reduced in protein, phosphorous, and sodium content; contain high-quality protein sources; be highly digestible; and provide adequate potassium, nutrient, and caloric density. In a recent study, feeding a diet appropriately modified in protein, phosphorus, lipids, and sodium was associated with stable renal function and delayed onset of uremia in dogs with moderate chronic renal failure.55 These dogs had a better perceived quality of life, lower risk of death, and prolonged survival (>13 months) compared with dogs fed a maintenance diet. In these studies other treatments appropriate to the stage and consequences of renal failure were initiated as needed, lending support to the long-term benefits of a carefully crafted and monitored treatment regimen for chronic renal diseases. Similarly, in 45 cats with mild to moderate renal failure followed for up to 2 years, cats fed a renal diet had significantly lower all-cause mortality rates and no uremic crises or renal mortality compared to cats fed a maintenance diet. Cats fed the diet formulated for renal disease also had reduced azotemia and acidosis during the study period.56 The composition and nutrient profiles for commercial renal diets are available in manufacturers’ product information and summarized in a review by Bartges and Brown.57Dietary supplements or homemade diets may be required to meet the needs of individual patients.
Management of Anorexia and Vomiting
The best dietary strategy is ineffective if the patient becomes anorectic, cannot consume adequate calories for energy needs, or is vomiting and intolerant of enteral feeding. Many metabolic consequences of renal failure may affect appetite, including hydration status, severity of uremia, degree of anemia, acidosis, secondary hyperparathyroidism, gastrointestinal complications, and electrolyte imbalances. In the sick, uremic animal, correction of dehydration, acidosis, electrolyte abnormalities, and gastrointestinal complications should be accomplished before attempting to introduce a therapeutic renal diet58 Supplementation of water-soluble vitamins and correction of anemia also may improve appetite. ACE inhibitors, some antimicrobials, and many other therapeutic agents can contribute to anorexia, and their potential benefit should be reviewed critically in intolerant patients.
In anorectic patients with chronic renal failure, a review of previous diets, dietary habits, and drug therapy is advised. As with all dietary changes, new diets should be introduced gradually, and small, more frequent meals may be preferable for many patients. Owners and nursing staff can tailor the feeding schedule and feeding environment to enhance appetite by avoiding hurried, noisy feeding or feeding in close association with painful or stressful procedures. Some animals respond to hand feeding or feeding during petting and socialization, especially in a quiet ward or outside the hospital area. Warming of food, moistening food, and ensuring easy access to food are practical methods of improving acceptance.58 Flavoring agents can also be added, including animal fat, bouillon, clam juice, tuna broth, brewer’s yeast, garlic, butter, or cottage cheese.37 An added benefit of flavored liquids is enhanced fluid intake, although broths high in sodium and phosphorus should be avoided. Supplementation of vegetable oils, margarine, cream, or complex sugars may be used to increase caloric intake.31 If oral ulcers that limit food intake are observed, application of xylocaine gels or cool tea flushes may be used to alleviate pain. Enteral feeding using esophagostomy or gastrostomy tubes are another option for managing chronic renal failure in dogs and cats. They allow easy administration of medications and fluids in addition to providing adequate nutrition to an anorectic or hyporexic patient.
Gastrointestinal effects of uremia include mucosal irritation from nitrogenous waste products, impaired gastrointestinal mucosal barriers, and hypergastrinemia. Central receptors for appetite and nausea also are affected by retained substances and increased parathyroid hormone (PTH) concentrations. Anorexia, vomiting, and diarrhea are common complications of advanced renal failure. In patients with chronic renal failure, sporadic vomiting, nausea, and anorexia may be alleviated by the administration of histamine blockers such as cimetidine (5 mg/kg orally, intramuscularly, or intravenously every 6 to 8 hours), famotidine (0.5 to 1 mg/kg orally every 12 to 24 hours), or ranitidine (0.5 to 2 mg/kg orally every 12 hours). Cimetidine inhibits hepatic metabolism of many drugs, including β-blockers and calcium channel blockers, and should be avoided in patients receiving these drugs. Alternately, administration of a proton pump inhibitor (omeprazole 0.7 to 1.5 mg/kg orally every 12 to 24 hours in cats or 0.5-1 mg/kg orally every 24 hours) may be effective in cases refractory to histamine blockers. The addition of sucralfate, a gastrointestinal mucosal protectant, may be useful for patients with severe gastritis or suspected gastrointestinal hemorrhage. Because sucralfate is most effective in an acidic stomach environment, other antiemetics or antacid medications should be given at least 30 minutes after administration of sucralfate when used concurrently. For refractory vomiting metoclopramide (0.2 to 0.4 mg/kg subcutaneously, intramuscularly, or orally every 8 hours) may be administered to improve gastric emptying and reduce centrally mediated nausea. Dolasetron is a serotonin receptor antagonist that has been used extensively in people to decrease nausea and vomiting associated with chemotherapy and anesthesia. Little published information is available about the efficacy and pharmacokinetics of this drug in dogs or cats, but its use in veterinary medicine is growing. It has potential use in managing nausea and vomiting in dogs and cats caused by chemotherapy; anesthesia; enteritis; and metabolic diseases, including renal failure. A dose of 0.6 mg/kg intravenously or orally every 24 hours is recommended to prevent nausea and vomiting, whereas a higher dose of 1 mg/kg intravenously or orally every 24 hours is recommended for treatment of clinical emesis and nausea. It can be used in combination with other antiemetics, including metoclopramide.59 Ondasetron, another serotonin receptor antagonist, was shown to be about twice as effective as metoclopramide in alleviating nausea and vomiting in uremic human patients.60 Maropitant, a novel synthetic nonpeptide neurokinin type 1 (NK1) selective receptor antagonist, prevents and treats emesis and is licensed for use in dogs. The dose of 1 mg/kg orally or subcutaneously every 24 hours for up to 5 days is recommended for dogs to manage nausea and vomiting caused by a variety of factors, whereas a higher dose is recommend for prevention of motion sickness.61 Maropitant has not been approved for use in cats. One published study concerning its use in cats demonstrated that it is safe and effective in reducing the incidence of vomiting induced in laboratory cats by xylazine administration or motion sickness.62
Misoprostol, a synthetic prostaglandin analog, inhibits gastric acid and pepsin secretion and has a cytoprotective effect on gastric mucosa. The drug may be useful in renal failure–induced gastritis at a dosage of 1 to 5 μg/kg orally every 6 to 8 hours. Transient gastrointestinal upset is a possible adverse effect of misoprostol administration that may be managed by adjusting the drug dosage and giving the drug with food.17 Anorexia or gastrointestinal complications of drug administration must be addressed quickly in patients with renal failure, however, because dehydration and renal decompensation can occur.
Management of Systemic Hypertension
In animals with chronic kidney disease, the afferent arteriole dilates, which leads to increased intraglomerular pressure. The kidney is susceptible to hypertensive damage, on account of both elevated systemic arterial blood pressure and intraglomerular pressure. In dogs there is a close association between elevated intraglomerular pressure and progressive renal injury. Systemic hypertension is observed in more than 60% of dogs and cats with renal disease, particularly in animals with glomerular disorders, renal vascular disease, and renal neoplasia.41 Multiple mechanisms may contribute to the development of hypertension in renal failure, including decreased glomerular filtration, impaired sodium and water handling, local activation of the renin–angiotensin–aldosterone system, and impaired production of renal vasodilatory substances. High systolic blood pressure (>163 mm Hg) in dogs at the time of diagnosis of chronic renal failure was associated with increased risk of developing a uremic crisis and dying, compared with dogs that had lower blood pressure.63 Controlling hypertension may decrease the rate of progression of chronic renal failure. Clinical signs of hypertension in small animals are usually manifestations of ocular complications, including blindness, retinal hemorrhages, retinal detachment, and glaucoma, but they may include cardiac failure, neurologic signs, hemorrhage, and effusions. Overt signs are often inapparent, however, and blood pressure recordings should be routinely monitored in patients with renal disease.
Moderate restriction of sodium intake is one step in management of mild systemic hypertension. Dietary sodium content of 0.1% to 0.3% sodium by dry matter is recommended for initial management.41,64 Most commercial “renal” diets provide appropriate sodium content, limiting sodium intake to 10 to 40 mg/kg per day. Sodium restriction should be gradual so as not to precipitate volume depletion. If necessary, additional sodium restriction may be accomplished by feeding homemade diets or diets formulated for cardiac disease.
Pharmacologic manipulation of blood pressure may be indicated in animals with moderate to severe hypertension (systolic blood pressure >180-200 mm Hg), clinical signs attributable to hypertension, or persistent hypertension despite sodium restriction. There is evidence that dogs with renal failure have great variability in blood pressure, and it may be appropriate to initiate antihypertension drugs in dogs with intermittently elevated blood pressure (>160/100 mm Hg).46 The choice of agent must be based on the potential risks and benefits in the individual animal and the clinician’s experience and preference. A variety of agents have been proposed for use in hypertension, including ACE inhibitors and calcium channel blockers, diuretics and β-blockers.
In dogs ACE inhibitors are the first choice for management of hypertension. Inhibition of angiotensin II production leads to decreased aldosterone secretion, decreased blood pressure, efferent arteriolar dilation, and reduced intraglomerular capillary pressure. In glomerular disease ACE inhibitors are helpful in controlling hypertension and minimizing proteinuria. In dogs with experimentally induced chronic renal insufficiency, enalapril was shown to decrease proteinuria and glomerular capillary pressure after 3 and 6 months of therapy, respectively. Dogs receiving enalapril had fewer glomerular and tubulointerstitial lesions than the placebo-treated group.65
Potential risks of ACE inhibitor administration include hypotension; decreased renal perfusion; hyperkalemia; gastrointestinal upset; and, rarely, myelosuppression or seizures. Excessive reductions in renal perfusion and GFR are most worrisome because they may lead to acute decompensation of renal failure. To prevent this complication, administration of ACE inhibitors is initiated at a low dosage while blood pressure, blood urea nitrogen, and creatinine concentration are measured. The drug may be slowly increased to an effective dosage. Starting dosages of enalapril and benazepril are 0.25 to 0.5 mg/kg orally per day. Benazepril is less likely to cause renal damage than enalapril and has been shown to decrease systolic blood pressure in dogs and cats. A dose of 0.5 to 1 mg/kg orally every 24 hours successfully decreased blood pressure in cats with experimentally induced renal disease, without decreasing GFR.66 Irbesartan (5 mg/kg orally every 12 to 24 hours) is an angiotensin II–receptor blocker that will also lower blood pressure in dogs.67 Further research is needed to determine whether all cats and dogs with chronic renal failure or renal insufficiency would benefit from ACE inhibitor therapy. This class of drugs may be renoprotective without lowering systemic blood pressure.
Calcium channel blockers such as diltiazem or amlodipine also are attractive agents for the management of hypertension in patients with renal failure. Amlodipine (0.625 to 1.25 mg/cat daily by mouth) has become the preferred agent for cats.68 In dogs that are nonresponsive to ACE inhibitors or in which the drugs are contraindicated, amlodipine (0.05 to 0.25 mg/kg orally every 24 hours) can be administered. Calcium channel blockers reduce blood pressure by peripheral vasodilatory effects; potency varies with the preparation. Calcium channel blockers increase peripheral resistance, leading to a decrease in blood pressure, but they also dilate afferent renal arteriole, which can be detrimental. There are some concerns about possible detrimental effects of calcium channel blockers, which were associated with exacerbation of renal injury, proteinuria, or both in studies of people and diabetic dogs. Newer classes of calcium channel blockers may offer increased renoprotective effects by dilating both the efferent and afferent renal arterioles.67 Calcium channel blockers also possess cytoprotective qualities that may be helpful in acute or chronic renal damage. Calcium channel blockers are negative inotropes and may cause hypotension, cardiac arrhythmias, and gastrointestinal upset in some patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree