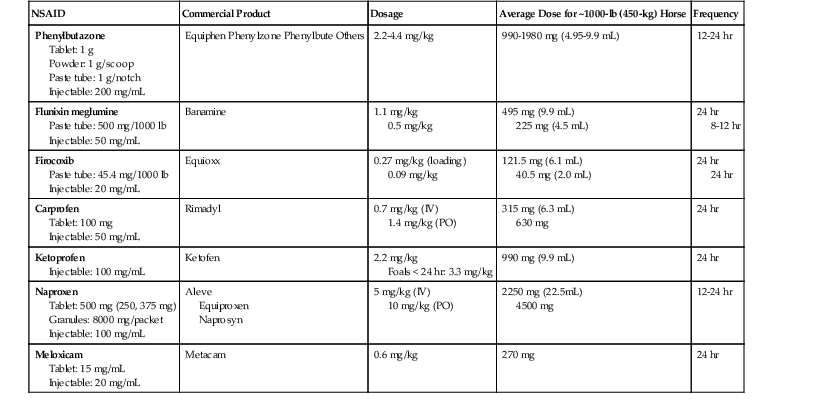
Brad B. Nelson, Laurie R. Goodrich Osteoarthritis (OA) or degenerative joint disease is a frequently encountered problem in horses and results in considerable costs to the equine industry. It is a progressive disease characterized by joint pain, inflammation, synovial effusion, limited range of motion, and progressive deterioration of articular cartilage. Osteoarthritis is a nonspecific term that may be used to include injury solely of the articular cartilage, but frequently, multiple structures associated with the synovial joint are simultaneously affected. A synovial joint is composed of two opposing layers of articular cartilage and underlying subchondral bone. The articular cartilage acts as a smooth gliding surface for joint mobility. Surrounding the joint is a synovial membrane, which is responsible for synthesizing synovial fluid, hyaluronan, and lubricin, which provide for viscosity and lubrication. The joint capsule is connected to the synovial membrane and has a fibrous portion composed of dense connective tissue, which is continuous with the periosteum or perichondrium of the surrounding bones. The joint capsule, collateral ligaments, surrounding musculotendinous units, and intraarticular ligaments (such as the cruciate ligaments in the stifle joint) provide stability of the joint. Proposed mechanisms of joint injury include traumatic arthritis, physiologic trauma secondary to inflammation, or athletic activity leading to injury. Traumatic arthritis is a broad term encompassing single or repetitive trauma to one or any combination of the above structures. A subclassification has been developed to clarify this type of injury. Type I is traumatic synovitis and capsulitis, without cartilage or surrounding soft tissue damage. Type II involves damage to articular cartilage or complete rupture of major supporting structures, such as collateral ligaments, and type III is posttraumatic arthritis, in which articular cartilage progressively deteriorates, accompanied by secondary changes in the surrounding soft tissues or bone. The second mechanism of OA is persistent inflammation. With persistent synovitis, secondary inflammatory changes within the joint may progress to OA, even with normal joint loading. The third mechanism of injury is repetitive high-impact loading of the normal joint during athletic activity, which results in repetitive microtrauma. Whatever the inciting cause, damage to articular cartilage and continued inflammation in the joint can lead to progression of disease. As a consequence, persistent OA or injury to the joint capsule may result in joint capsule fibrosis and decreased range of motion. Horses with clinical OA have various presentations. Some horses have a significant degree of lameness, but others may have no lameness. Synovial effusion may be palpable, but the volume of effusion does not directly correlate to severity of disease, nor does it confirm OA. Flexion tests can increase suspicion of joint injury, but these tests do not rule out injury or confirm it to a specific joint. Intraarticular analgesia is the most specific method of localizing pain to a joint, with improving or resolving lameness seen following injection. Synovial fluid may be abnormal, although the severity of inflammation or degree of structural change is not at all indicated by synovial fluid values. Normal synovial fluid is clear with a light yellow hue and has good viscosity. White blood cell counts in normal fluid are typically less than 500 leukocytes/µL, with more than 90% mononuclear cells and less than 10% neutrophils. Total protein concentration is typically less than 2.5 g/dL. Osteoarthritis typically causes mildly high leukocyte counts (<1000/µL) and total protein (<3.5 g/dL), but the cell populations do not change, compared with the changes seen with septic arthritis. Synovial biomarkers are more commonly used in research settings than in clinical medicine and can suggest alterations in joint anabolism and catabolism as are present with OA. Osteoarthritis can be diagnosed by multiple imaging methods. Radiography is the most common (although it is extremely insensitive with early OA) and may reveal one or several of the following changes: periarticular osteophytes, subchondral bone sclerosis or lysis, loss of joint space that may be asymmetrical (suggesting cartilage loss), osteochondral fragmentation, or ankylosis. Ultrasonography may reveal synovial effusion, periarticular osteophytes, synovitis, capsulitis, damage to surrounding soft tissue structures, and in some instances, thinning cartilage or defects within subchondral bone. Computed tomography and contrast arthrography can also demonstrate changes in bone and intraarticular ligaments encountered in osteoarthritis. Magnetic resonance imaging is the most sensitive method of diagnosing cartilage injury and can simultaneously evaluate surrounding structures. Both computed tomography and magnetic resonance imaging are more sensitive in identifying lesions than radiography, because of the dimensional aspect of imaging. Nuclear scintigraphy can be useful, but as OA becomes chronic, this technique may have a lower yield because of lack of specificity, and another type of imaging is typically required to confirm the diagnosis. There are many treatment options for OA, including systemic, local intraarticular, and topical medications; administration of nutraceuticals; surgery; and physical therapy and rehabilitation. The focus of this chapter is on the medical management of OA. Because osteoarthritis is a progressive disease and not curable, early diagnosis and intervention typically allow for the best chance for return to athletic function. The main goals of medical treatment are to decrease pain and minimize further joint deterioration. These therapies can be classified into symptom-modifying osteoarthritis drugs (SMOADs) and disease-modifying osteoarthritic drugs (DMOADs) and can be a combination of both. Symptom-modifying drugs improve the signs of OA (including lameness), are pain modifying, and include antiinflammatory properties. Disease-modifying drugs may not result in clinical improvement in lameness but have chondroprotective properties and increase anabolic effects and decrease catabolic effects within the joint. Systemic treatments, although nonspecific, are common for treating OA. Commonly, these are the first line of treatment because of their ease of administration and availability. It is common to administer systemic treatments while also using local therapies, which will be discussed later. The most frequently used systemic treatments include nonsteroidal antiinflammatory drugs (NSAIDs), polysulfated glycosaminoglycans, hyaluronan, and sodium pentosan polysulfate. Nonsteroidal antiinflammatory drugs have been administered for many decades because of their analgesic, antiinflammatory, and antipyretic properties. The mechanism of action for NSAIDs is inhibition of an enzyme that converts arachidonic acid to prostaglandins and thromboxanes. Cyclooxygenase (COX) is the primary enzyme and has two primary isoenzymes: COX-1 (the constitutive form of the enzyme) and COX-2 (the inducible form). COX-1 regulates prostaglandins involved in normal cellular processes, whereas COX-2 is thought to be primarily responsible for inflammatory responses. As a result, COX-2–inhibiting medications are thought to treat inflammation without compromising normal physiologic processes. However, COX-2 also is constitutively formed in some tissues; thus, the “COX-1 is the good isoenzyme and COX-2 is the bad isoenzyme” statement is not completely accurate. A description of commonly used NSAIDs is provided (Table 187-1). Phenylbutazone, which is a nonselective COX-inhibiting medication (i.e., inhibits both isoenzymes), is one of the most commonly administered NSAIDs because of its low cost, ease of administration, and relatively few adverse effects. A dosage of 4.4 mg/kg, given every 12 hours, is safe and, in some instances, is used as a loading dose for the first 2 days of treatment. However, it is strongly recommended to decrease the dose after a few days to minimize the possibility of toxicosis in the form of renal papillary necrosis, right dorsal colitis, or gastrointestinal ulceration. Oral cavity ulcers may also develop in some cases. As a general rule, the minimally effective dose should be used to limit potential adverse effects, and a dosage of 2.2 mg/kg given twice daily is relatively safe. Similar to phenylbutazone, flunixin meglumine is a nonselective COX inhibitor. The drug is rapidly absorbed, with peak plasma concentrations achieved in 30 minutes and a plasma half-life of 1.6 hours. The greatest effect is typically seen 2 to 16 hours after administration and may persist for up to 30 hours. Oral administration at 1.1 mg/kg every 24 hours is typically safe, but a similar toxicity profile can be seen as was described for phenylbutazone if this drug is not used judiciously. Smaller doses (0.5 mg/kg) are used for antiinflammatory effects and are not as effective for severe pain. No studies exist that demonstrate superiority of flunixin meglumine over phenylbutazone in horses with musculoskeletal injury, but anecdotally, many practitioners favor phenylbutazone for these cases. Firocoxib1 is a COX-2 inhibitory drug labeled for treatment of OA in horses. It is formulated as an oral paste or injectable solution. A loading dosage of 0.27 mg/kg is recommended and is followed by a dose of 0.09 mg/kg every 24 hours. Bioavailability is about 79%, and peak plasma levels of the drug are reached at 3.9 hours. The elimination half-life is 30 hours. Toxicosis with this medication is rare. Even when firocoxib was administered at five times the recommended dose for up to 92 days, study horses had no clinical signs of toxicosis, although histologic changes in the kidneys were reported in some horses. The injectable solution is viscous compared with other injectable medications and precipitates when mixed with any aqueous solution. The higher cost of this formulation often precludes its use. The canine formulation (Previcox) was used before licensing of the equine product, but no studies exist on its bioavailability or safety in horses, and its use in horses is extralabel. Carprofen is an NSAID approved for use in Europe. It is formulated as an injection or tablet. Carprofen also has more COX-2 inhibition than COX-1 inhibition and is well tolerated when given at twice the recommended dose for 14 days. This dose provides adequate analgesia for 11.7 hours, with a half-life of 18 to 22 hours. It is not recommended for intramuscular use because of injection site swelling. Ketoprofen has a lower risk for toxicosis compared with phenylbutazone, but reportedly has less efficacy for treatment of musculoskeletal pain. Higher doses are used in foals younger than 24 hours to account for the higher volume of distribution. Oral formulations are not bioavailable. The cost of this medication typically makes its use reserved for foals. Naproxen is a nonselective COX inhibitor. Bioavailability is about 50%, and peak plasma levels are reached at 2 to 3 hours; half-life is approximately 4 to 5 hours. Comparisons of efficacy to phenylbutazone and flunixin meglumine have not been made. No signs of toxicosis were observed when naproxen was administered at three times the recommended dose for 3 weeks in one study. Meloxicam has higher COX-2–inhibiting activity than COX-1–inhibiting activity and is available for horses in Europe. It is administered at 0.6 mg/kg every 24 hours for a maximum of 14 days. Adverse effects appear to be similar to those seen with other NSAIDs. Other NSAIDs that have been used in treating OA in horses include acetylsalicylic acid (aspirin), meclofenamic acid,2 and vedaprofen. The reader is referred to the footnotes and Suggested Readings at the end of the chapter for information regarding these medications. Polysulfated glycosaminoglycans3 (PSGAGs) are a mixture of glycosaminoglycans, a compound present in the extracellular matrix of articular cartilage. Although the product has been administered to both humans and equids with OA, the exact mechanism of action is unknown. It is theorized that PSGAGs form complexes, which are deposited into cartilage. Benefit appears to be a result of antiinflammatory effects and inhibition of prostaglandins and other degradative enzymes that contribute to OA. Administration of PSGAGs has also been documented to promote stimulation of hyaluronan and collagen synthesis. When administered intramuscularly, the compound appears to produce minimal side effects. Dosage regimens vary, but multiple studies have failed to provide scientific evidence for its intramuscular use in horses with OA; however, many practitioners and horse owners use this medication with anecdotal success.
Treatment of Joint Disease
Introduction of Joint Disease and Importance
Pathophysiology of Joint Disease
Diagnosis of Joint Injury
Therapy
Systemic Treatments
Nonsteroidal Antiinflammatory Drugs
Phenylbutazone.
Flunixin Meglumine.
Firocoxib.
Carprofen.
Ketoprofen.
Naproxen.
Meloxicam.
Polysulfated Glycosaminoglycans
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Treatment of Joint Disease
Chapter 187
Only gold members can continue reading. Log In or Register to continue