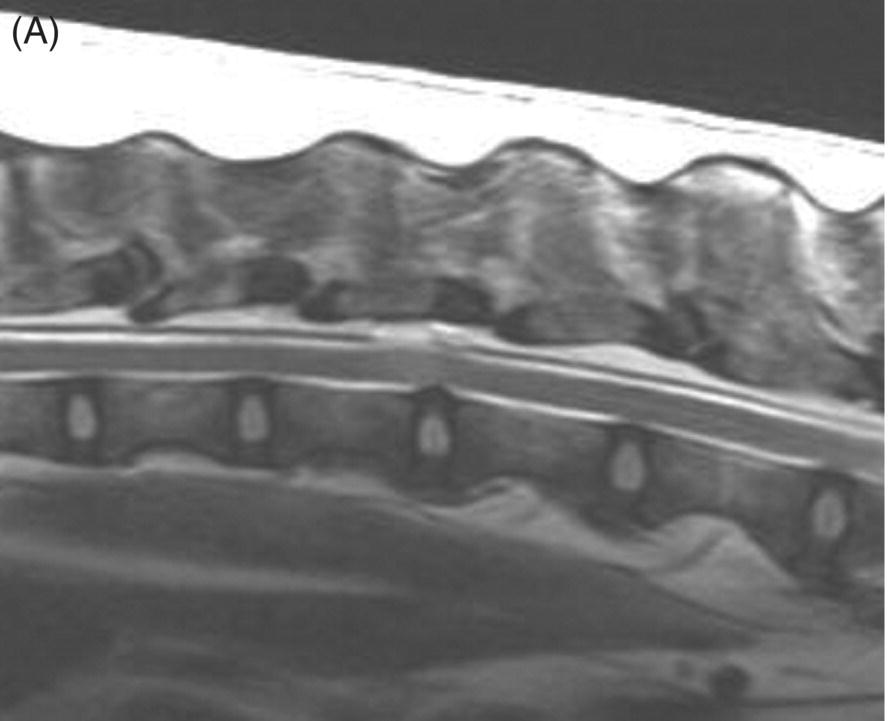
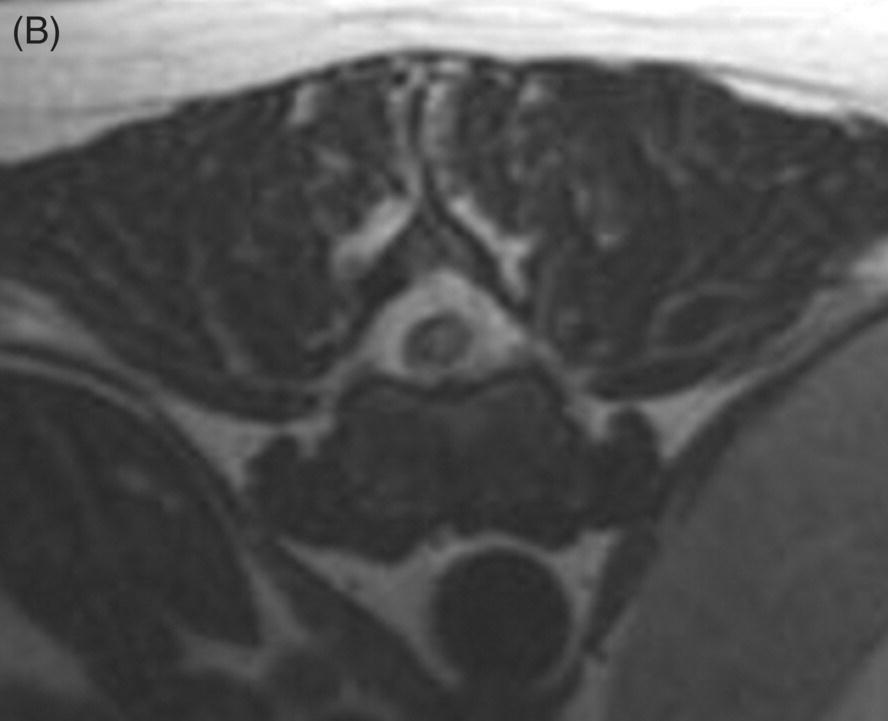
13 Luisa De Risio, William B. Thomas, and James M. Fingeroth Traumatic disc extrusions (TDE) may occur when an otherwise healthy intervertebral disc is subjected to excessive force such as during vigorous exercise (such as running and jumping) or trauma. A sudden increase in intradiscal pressure can cause rapid projection of hydrated nucleus pulposus (NP) toward the spinal cord through a tear in the annulus fibrosus. This results in spinal cord contusion and may or may not result in persistent spinal cord compression. The term acute noncompressive nucleus pulposus extrusion (ANNPE) has been proposed to indicate when the extruded NP contuses the spinal cord and then dissipates within the epidural space, causing minimal to no spinal cord compression [1]. Other terms to indicate this condition include traumatic disc prolapse, dorsolateral intervertebral disc “explosion,” and high-velocity–low-volume disc extrusion [2–4]. Some authors have also used the term Hansen type III intervertebral disc disease [5]. However, Hansen’s terminology only included two types, and type III extrusions were originally described by Funquist as extension of disc material “like a carpet over several vertebrae” [6]. Most recently, the term TDE has been used to indicate extrusion of either degenerated or nondegenerated intervertebral disc material following trauma to the spinal region [7]. ANNPE has been reported in several canine breeds and in a few cats [1–9]. Compressive hydrated nucleus pulposus extrusion (HNPE) has been reported in dogs and has been mainly characterized by ventral midline spinal cord compression [10]. Rarely, TDE can result in laceration of the dura mater and in some cases penetration of the spinal cord parenchyma [11–20]. Subarachnoid–pleural fistula has been reported in one dog following TDE at T11–T12, resulting in dura mater laceration and separation of the hypaxial muscle fascial planes and the parietal pleura [21]. TDE can also occur in association with vertebral fracture/luxation. In the authors’ experience, this is infrequent in dogs and cats, and neurologic deficits are usually due to bony displacement or hematoma. However, in human patients, TDE has been reported in up to 40% of all fracture dislocations of the cervical spine [22–24]. The clinical presentation of dogs and cats with ANNPE is characterized by peracute onset of myelopathy that is nonprogressive after the first 24 h. Lateralization of neurological deficits has been reported in 62% of dogs and in two of the three cats reported [1, 8, 9]. Discomfort and hyperalgesia during palpation of the affected spinal segments have been described in 57% of dogs [1]. This is the most helpful clinical finding to differentiate ANNPE from fibrocartilaginous embolic myelopathy (see Chapter 9), although occasionally animals with fibrocartilaginous embolic myelopathy may have spinal pain or discomfort upon palpation in the first 24–48 h after onset. Male dogs seem to be affected more commonly with TDE than females. Median age at diagnosis in dogs has been reported as 6.7 years [1]. The T3–L3 spinal cord segments and in particular the T12–T13, T13–L1, and L1–L2 intervertebral disc spaces are most commonly affected [1, 25]. A transient decrease in magnitude of the flexor withdrawal reflex in the pelvic limbs has been reported in half of dogs with thoracolumbar ANNPE examined within hours to a few days after onset of signs and is probably due spinal shock [1]. The age at disease onset in the three cats reported with ANNPE was 16 months, 2 years, and 5 years, respectively [4, 8, 9]. Two cats were female and one was male. Breeds included domestic short and long haired and Siamese. Affected spinal cord segments were C2–C3, C3–C4, and L5–L6, respectively [4, 8, 9]. The clinical presentation of the cat and few dogs reported with TDE resulting in dura mater laceration and penetration of the spinal cord parenchyma was similar to the one described for ANNPE [11–20]. The thoracolumbar junction is most commonly affected, although cervical intervertebral discs can be affected [11–20]. To date, compressive HNPE has been reported in the cervical spine of 10 dogs [10]. In all but 1 of these 10 dogs, the onset of neurological signs was not associated with any type of physical activity (running, jumping, or playing) or traumatic event (road traffic accident). In the remaining dog, the onset of clinical signs appeared while running, without any witnessed traumatic event. All dogs had acute (<24 h) symmetric nonambulatory tetraparesis or tetraplegia. Respiratory dysfunction was observed in 33% of dogs. The median age at diagnosis was 9 years (range, 8 to 13 years). Most dogs were nonchondrodystrophic and male. The most commonly affected IVD space was C4–C5 followed by C3–C4 [10]. The authors have observed compressive HNPE in the cervical and thoracolumbar spine of dogs following trauma or strenuous exercise. In one study on 31 dogs with TDE [7], including dogs with intervertebral disc degeneration, 9 dogs (21%) had concurrent spinal cord compression and the remaining 22 (71%) had no spinal cord compression. Mean age was 6.3 years (range, 6 months to 15 years) and mean body weight was 14.2 kg (range, 2.5–32 kg). The most common sites for TDE (compressive and noncompressive) were the cervical and the thoracolumbar (T12–L4) regions of the spinal column. In addition to TDE, 7 of 9 dogs with spinal cord compression and 7 of 22 dogs without spinal cord compression had evidence of generalized IVD degeneration. Dogs with TDE and spinal cord compression were significantly older and more likely to be chondrodystrophic and have generalized IVD degeneration than were dogs with traumatic IVD extrusion without spinal cord compression. Body weight and initial neurologic grade did not differ significantly between dogs with and without spinal cord compression [7]. The antemortem diagnosis of TDE is based on clinical features (acute onset of nonprogressive myelopathy following trauma or strenuous exercise) and MRI. The MRI findings of ANNPE include a focal area of T2 hyperintensity within the spinal cord overlying a narrowed intervertebral disc space, with absent or minimal spinal cord compression. There is decreased volume and signal intensity of the affected NP on T2-weighted images. Extraneous material or signal change may be evident in the epidural space dorsally [1, 25] (Figure 13.1). These MRI findings can help to differentiate ANNPE from fibrocartilaginous embolic myelopathy (see Chapter 9) and to identify penetration of NP material within the spinal cord [16, 17]. Figure 13.1 MRI of a dog with noncompressive traumatic disc extrusion. A 9-year-old neutered male Labrador suffered peracute left pelvic limb monoparesis while playing with a ball. When correcting for overshooting the ball, his left pelvic limb appeared to slide beneath him and he yelped. From that time, he was unable to use his left pelvic limb. Neurologic examination revealed severe paresis of the left pelvic limb.(A) Sagittal T2-weighted fast spin echo (FSE) MRI. There is mild swelling and ill-defined intramedullary hyperintensity of the spinal cord overlying the T13–L1 intervertebral disc space. There is disruption of the epidural space dorsally to the spinal cord. The size of the T13–L1 intervertebral nucleus pulposus appears slightly smaller than the volume adjacent discs. (B) Transverse T2-weighted FSE MRI. The intramedullary hyperintensity is left sided and immediately dorsal to the disrupted dorsal annulus on transverse T2-weighted images There is disruption of the epidural space dorsally and laterally on the left side (Source: Luisa De Risio ©).
Traumatic Disc Extrusions
Pathophysiology
Clinical presentation
Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


