CHAPTER 28 Toxicologic Considerations in the Young Patient*
Relevant Physiologic Differences in Pediatric Patients Relative to Adults
Pups and kittens may be exposed to toxins through various routes: ingestion (including the ingestion of mother’s milk), topical exposure, inhalation, and ocular exposure. In dogs and cats, the first 12 weeks of life is a time of significant developmental changes. Physiologic alterations associated with these maturation stages can predispose the pediatric patient to be more susceptible to adverse reactions. All aspects of drug disposition—absorption, distribution, metabolism, and excretion—are affected by these dramatic developmental changes as the neonate matures (Table 28-1).
TABLE 28-1 Altered xenobiotic disposition in pediatric patients
| Alteration | Impact |
|---|---|
| Increased intestinal permeability | Increased oral uptake, toxic plasma concentrations |
| Increased gastric pH | Increased oral uptake of weak bases and acid-labile compounds, prolonged and elevated plasma levels, toxic plasma concentrations |
| Altered peristalsis (decreased gastric emptying time) | Decreased absorption, lower plasma levels of toxin |
| Decreased plasma proteins | Toxin may accumulate, leading to more unbound compound and thus a potentially longer half-life |
| Decreased body fat | Increased plasma levels; decreased accumulation of lipid-soluble toxins |
| Increased total body water (more extracellular fluid) | Decreased plasma concentrations, longer half-life |
| Increased uptake of volatile gases | High plasma concentrations, increased response and toxicity |
| Increased dermal absorption | Higher or prolonged plasma exposure levels, toxicity increased |
| Immature P-glycoprotein system | Poor ability to clear toxins with this system |
Management of Toxicosis
Toxicologic History
There is an art to acquiring a good toxicologic history. If the history is to provide any type of working diagnosis, the veterinarian’s interview must be meticulous, caring, and thorough in scope. The veterinarian must be a calming influence if a reliable account of events is to be obtained. Specific criteria characteristic of a toxicologic history include what poison or poisons are involved, when the exposure occurred, how much poison the animal was exposed to, and the route of the exposure It is particularly relevant to inquire about the entire litter, particularly if they are still together. Additionally, what is the mother’s condition and is there a possibility that she has been exposed to a compound that could be a problem for her offspring (regardless of whether she is exhibiting clinical signs of toxicosis)? Obtaining packaging containers for known exposures is significant in identifying the compounds involved and quality available to be ingested. Some aids in obtaining an organized history are outlined in Boxes 28-1 and 28-2.
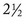 months of age. If normal levels of body fluids and electrolytes are maintained, pediatric renal tubular resorption is equivalent to that in adults. In this pediatric renal environment, water-soluble toxins have decreased clearance and extended half-lives. An example of this phenomenon is the recommendation that pediatric patients require higher doses (as a result of the increased volume of distribution) and longer dosing intervals (as a result of increased distribution and decreased clearance) of gentamicin. One can anticipate alterations in excretion in sick or dehydrated pediatric patients.
months of age. If normal levels of body fluids and electrolytes are maintained, pediatric renal tubular resorption is equivalent to that in adults. In this pediatric renal environment, water-soluble toxins have decreased clearance and extended half-lives. An example of this phenomenon is the recommendation that pediatric patients require higher doses (as a result of the increased volume of distribution) and longer dosing intervals (as a result of increased distribution and decreased clearance) of gentamicin. One can anticipate alterations in excretion in sick or dehydrated pediatric patients.


