CHAPTER 16 Viral Infections
Neonatal and puppy-kitten mortality rates (animals dying before 1 year of age) are often used as clinical indicators of the general level of health in the community, including the level of vaccination that is used in the area (Table 16-1). Infectious diseases are a major threat to the health of neonatal animals and may contribute to the mortality rate. Neonatal puppies and kittens depend in large part on the presence of sufficient maternal immunity, which provides short-term, passive immunity to protect them against microbial pathogens in the neonates’ environment. The severity of clinical symptoms (disease) in puppies and kittens that may result from infectious microorganisms depends on several key factors (Box 16-1). In addition to current maternal passive immunity, they include preexisting maternal infection status, maternal and neonatal nutrition, neonatal thermoregulation, concurrent neonatal infections and parasitism, and hereditary defects of the immune system.
TABLE 16-1 Effects of population (herd) immunity* on neonatal puppy and kitten survival
| Effects on | High survivability | Low survivability |
|---|---|---|
| Herd immunity | Increased | Decreased |
| Maternal immunity | Increased | Decreased |
| Colostral immunity | Increased | Decreased |
| Maternal/cohort shedding | Decreased | Increased |
| Survival of neonates | Increased | Decreased |
* Defined as 85% or greater of the population that are immune. Immunity is attained by regular vaccination boosters and/or continual boosters from natural infections from subclinical carrier animals.
BOX 16-1 Factors influencing the severity of clinical symptoms in puppies and kittens
Modified from Root Kustritz MV: Neonatology. In Root Kustritz MV (ed): Small animal theriogenology, St. Louis, 2003, Elsevier, p. 283.
Control of neonatal viral infections involves screening for preexisting infections and vaccination (when available) of the dam, minimizing potential exposure during critical periods (Table 16-2) and ensuring adequate colostral intake by the neonates (see Chapter 14). The critical periods are preconception, pregnancy, periparturient, and postnatal. The outcome of infection during these periods depends on previous individual animal immunity and viral challenge from the environment.
Specific Viral Diseases of Pediatric Canine Patients
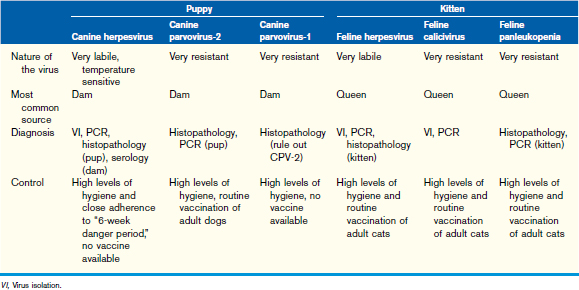
Canine Parvoviruses (CPV-2, CPV-1)
CPV-2
Infection is spread and acquired by the fecal-oral route. During the first 2 days after ingestion, viral replication occurs in the oropharynx and local lymphoid organs. Viremia from 3 to 4 days postinfection spreads virus throughout the body. Viremia is usually terminated when virus-neutralizing antibodies (IgG) are generated, usually 6 to 9 days postinfection. Clinical symptoms are most severe in puppies and may occur up to age 12 months. Parvoviral enteritis may present acutely or peracutely with anorexia and depression followed by vomiting and profuse, usually hemorrhagic, diarrhea. Newer variants of CPV-2 may cause a mild nonhemorrhagic diarrhea. Pyrexia, depression, anorexia, and dehydration are commonly observed. Intestinal damage in the rapidly developing crypt cells permits bacterial colonization that may result in endotoxic shock characterized by hypothermia, disseminated intravascular coagulation, and jaundice. Mortality rate may be as high as 25% and is a consequence of dehydration, endotoxic shock, electrolyte imbalances, and secondary bacterial infections (see Chapter 15). Mild or subclinical infections are common, especially in dogs aged more than 6 months.
Although there is no specific treatment for CPV-2, viral-bacterial enteritides generally consist of treating dehydration, sepsis, and acidosis/electrolyte imbalances. Intravenous balanced electrolyte solutions, systemic antibiotics (see Chapter 27), and antiemetic therapy may be indicated if vomiting is a significant component of the symptoms. Control measures include disinfection to reduce (dilute) the viral challenge load and immunization with modified live CPV-2 vaccines of adult dogs in the population and the susceptible puppies (Table 16-3). The role of immunization is twofold: the first is protection of individual puppies, and the second is to hyperimmunize adults to minimize viral shedding.
Canine Adenoviruses
The canine adenoviruses (CAV) consist of two predominant serotypes, CAV type 1 and type 2 (CAV-1 and CAV-2). CAV-1 is predominately multisystemic and causes hepatocellular necrosis and vasculitis. The virus is moderately resistant in the environment but susceptible to heat (steam cleaning) and disinfection by quaternary ammonium compounds. Dogs may carry this virus subclinically despite high levels of neutralizing antibodies (immune carriers, see Chapter 14). The antigenically related CAV, CAV-2, is predominately associated with upper respiratory tract disease. It is shed for 8 to 9 days postinfection.
Canine Herpesvirus
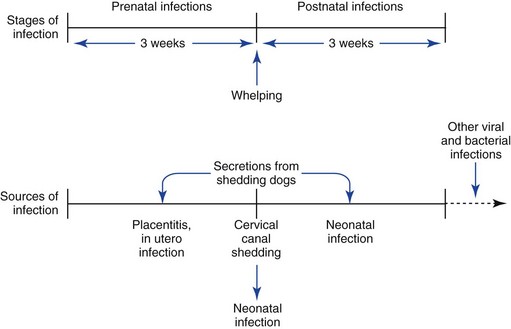
CHV has been recognized as one of the most common and virulent viral infections of neonatal puppies. Recent reports have indicated that this virus may be carried subclinically by up to 70% of some canine populations. As with other herpesviral infections, in particular, feline herpesvirus (see this chapter), CHV can be exacerbated following periods of physiologic, hormonal, and nutritional stress. CHV has several components that are important in control because there is no vaccine available in the United States. The virus is shed predominately in oronasal secretions from carrier dogs to susceptible dogs. This knowledge has been the basis for establishing the 6-week danger period, which includes the 3 weeks before whelping and 3 weeks postwhelping (Figure 16-1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree