Togaviridae
Abstract
This chapter describes the properties of togaviruses, specifically alphaviruses and features of the diseases they cause in animals.
Keywords
Alphavirus; Togaviridae; Togavirus
Viruses included in the two genera of the family Togaviridae possess a lipid envelope (or cloak: “toga”) surrounding an icosahedral capsid. The genus Rubivirus includes only rubella virus, the cause of rubella (“German measles”) in humans (Table 28.1). The viruses in the genus Alphavirus are predominantly arboviruses (viruses transmitted by arthropod vectors) and have the capacity to replicate in both mosquitoes and vertebrates. Exceptions to the requirement for mosquitoes in virus transmission include the salmonid alphaviruses that cause disease in several species of fish, and an alphavirus that infects southern elephant seals (Mirounga leonine). For both of these viruses the presence of the virus within lice (Lepeophtheirus salmonus for salmonid alphavirus and Lepidohthirus macrorhini for southern elephant sea lion virus) might also suggest an arthropod-borne cycle of infection, although this remains conjectural. Only the alphaviruses will be considered further, so in the context of this chapter “togavirus” is synonymous with “alphavirus.”
Table 28.1
Two genera: Alphavirus, arthropod-borne viruses, and Rubivirus, rubella virus (an exclusively human pathogen)
Virions are spherical, uniform in appearance, enveloped, 70 nm in diameter, and consist of an envelope with fine glycoprotein spikes surrounding an icosahedral nucleocapsid, 40 nm in diameter
The genome is a single molecule of linear, positive-sense, single-stranded RNA, 9.7–11.8 kb in size; the 5′ end of the genomic RNA is capped, whereas the 3′ end is polyadenylated
Genomic RNA is infectious
The 5′ two-thirds of the genome encodes nonstructural proteins; the 3′ one-third encodes the structural proteins, which are transcribed from a 26S subgenomic mRNA
Virions contain two (or three) envelope glycoproteins E1, E2, and E3, which form the spikes, and a nucleocapsid protein, C
Replication occurs in the cytoplasm, and maturation occurs via budding from the plasma membrane
Terrestrial alphaviruses are widely distributed throughout the world, and include several important pathogens of humans and/or animals. Individual alphaviruses typically exist in geographically restricted areas and habitats that are defined by transmission cycles that involve specific mosquito and vertebrate hosts that contribute to virus persistence, geographic distribution, overwintering, and amplification. More than one mosquito species is usually involved in the transmission cycle of individual viruses—these mosquito vectors become persistently infected and can transmit the virus at all subsequent feedings on their vertebrate hosts. Thus, the survival of alphaviruses in a given region depends on the presence of both competent vectors (mosquitoes) and vertebrate hosts (eg, birds, rodents, and monkeys) that develop productive (viremic) infection but with little disease (subclinical or asymptomatic infection). With the exception of Venezuelan equine encephalitis, chikunkungunya, and perhaps Ross River viruses, domestic animals and humans are “dead-end” hosts that are not involved in primary enzootic transmission cycles in nature, although they can serve as critical amplifying hosts that contribute to geographic extension and disease outbreaks. For example, a mosquito–horse–mosquito transmission cycle is responsible for explosive spread during epizootics of Venezuelan equine encephalitis. The host range for many alphaviruses is extensive, and may be restricted only by the feeding preferences of their insect (mosquito) hosts. Important alphavirus pathogens of vertebrate animals include eastern, western, and Venezuelan equine encephalitis and related viruses (collectively designated as the equine alphavirus encephalitides), and Getah virus. Although the pathogenic significance to animals of other alphaviruses is largely undefined, particularly in wildlife species, several alphaviruses in addition to those just listed are important zoonotic pathogens: specifically, Sindbis virus and a group of viruses related to Semliki Forest virus, including chikungunya, o’nyong-nyong, Ross River, Barmah Forest, and Mayaro viruses. With regard to disease in mammals, alphaviruses can be classified into three groups: (1) those that cause neurologic disease (encephalitis or encephalomyelitis); (2) those that cause a febrile illness with polyarthritis; (3) those that cause no apparent disease.
The alphaviruses have been historically grouped according to their geographic localization in the New World and Old World. The equine alphavirus encephalitides group is found exclusively in the New World, whereas the Semliki Forest and Sindbis virus groups are predominantly Old World viruses. Migratory birds have traditionally been thought to play a significant role in the local dispersal and global distribution of alphaviruses. It has been recently proposed that alphaviruses originated in the southern oceans and were then dispersed throughout both the New and Old Worlds.
Properties of TOGAVIRUSES
Classification
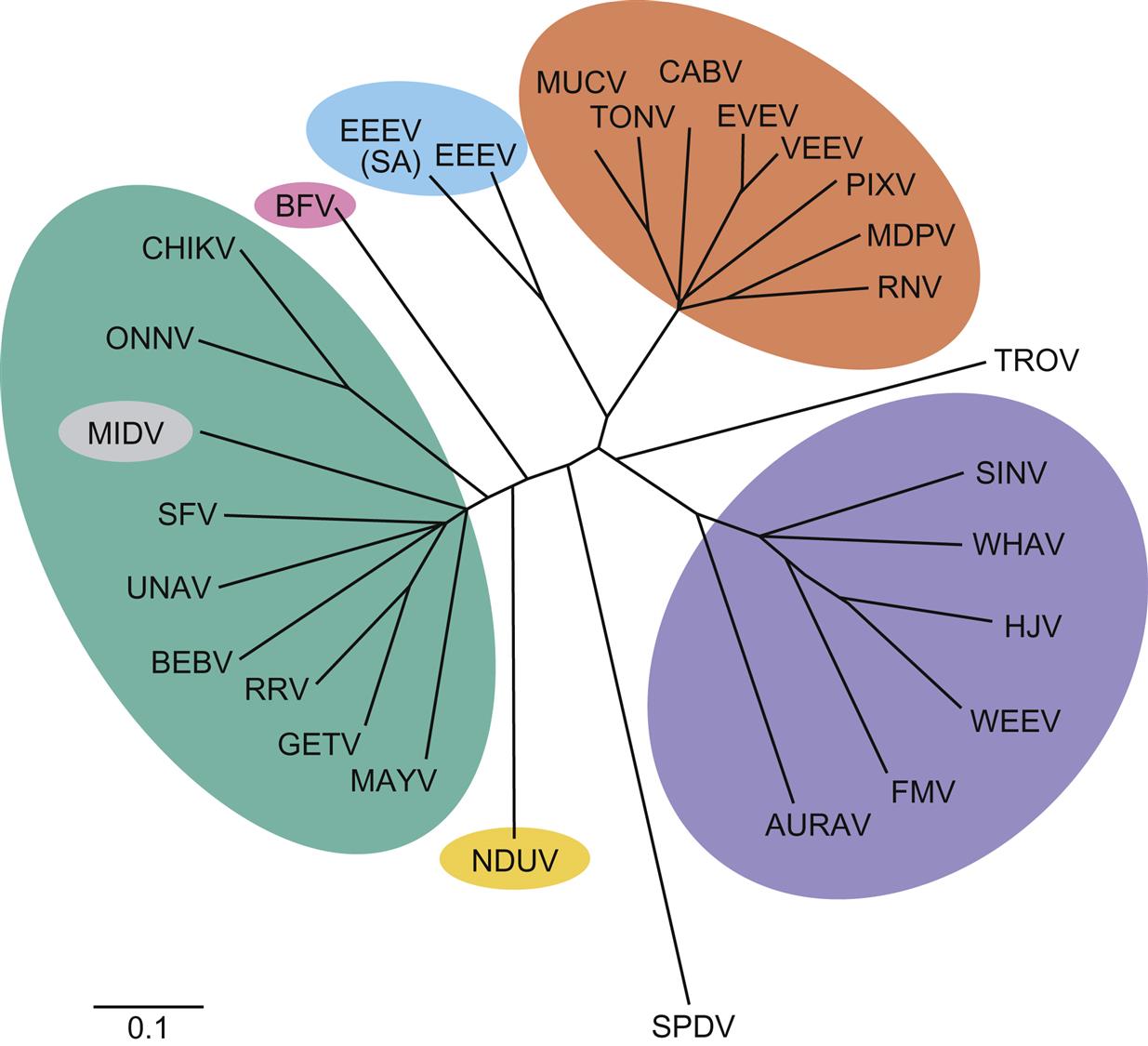
The family Togaviridae includes two genera, Alphavirus and Rubivirus. All viruses in the family Togaviridae that are either animal pathogens or zoonoses are included in the genus Alphavirus, thus only properties of alphaviruses will be described. The alphaviruses are further segregated into either New World or Old World groups: New World alphaviruses (eg, eastern equine encephalitis virus, western equine encephalitis virus, and Venezuelan equine encephalitis virus) are distributed across the Americas and cause encephalitis in equines and humans, whereas Old World alphaviruses (eg, Sindbis virus, chikungunya virus, o’nyong-nyong virus, Mayaro virus, Ross River virus, Barmah Forest virus, and Semliki Forest virus) are present in Europe, Asia, Australia, and portions of Africa and cause fever, rash, and arthritis in humans. Ross River, Sindbis, and chikungunya viruses have all been occasionally associated with human encephalitis. The alphaviruses can be further divided into seven antigenically related complexes that typically diverge from one another markedly as shown by phylogenetic analyses (Fig. 28.1).
Virion Properties
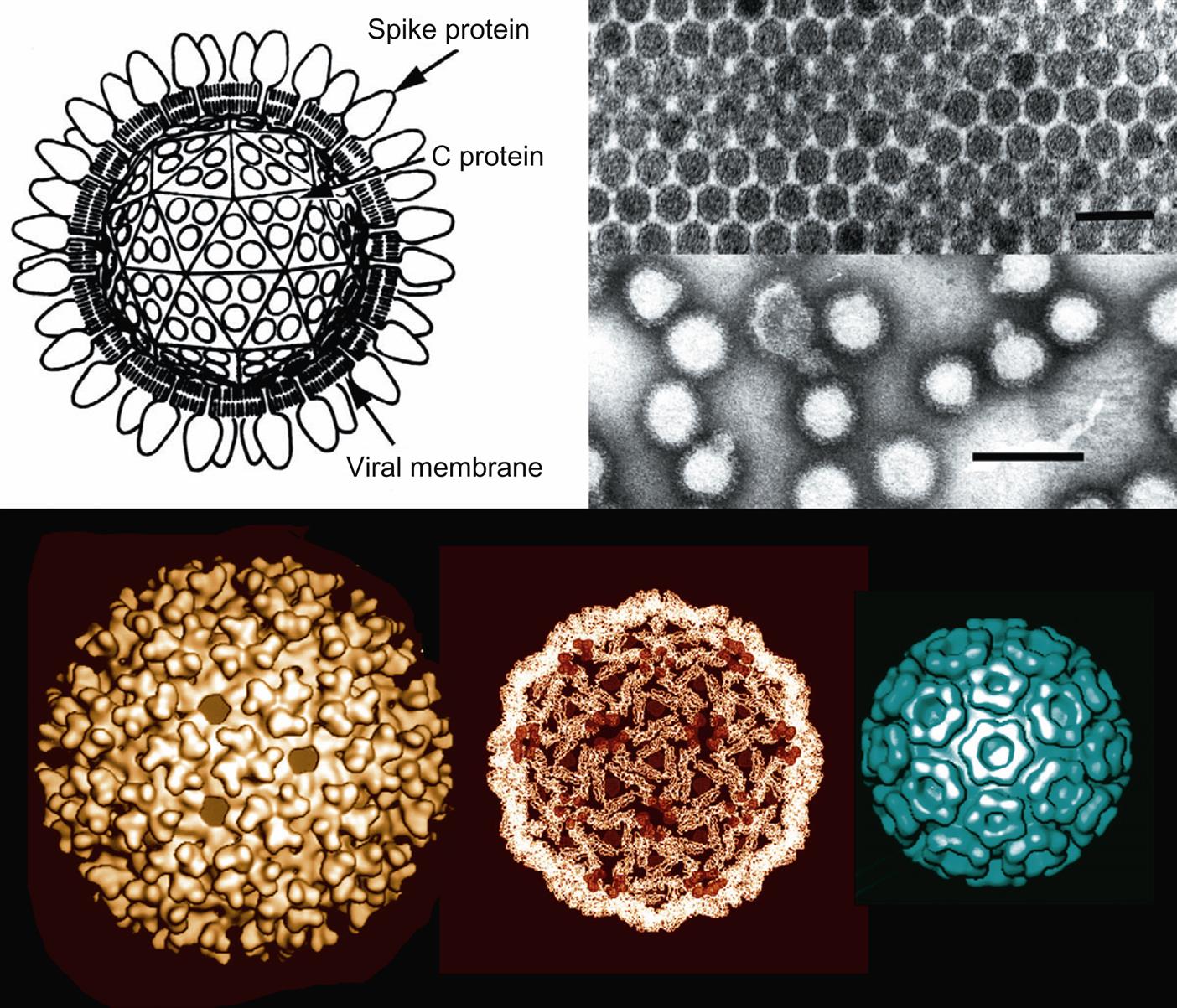
Alphavirus virions are spherical, uniform in appearance, enveloped, and 70 nm in diameter. Virions consist of a lipid envelope with fine spikes surrounding an icosahedral nucleocapsid that is 40 nm in diameter (Fig. 28.2). The spikes are composed of heterodimers of the E1 and E2 glycoproteins that are organized in a (T=4) icosahedral lattice consisting of 80 trimers.
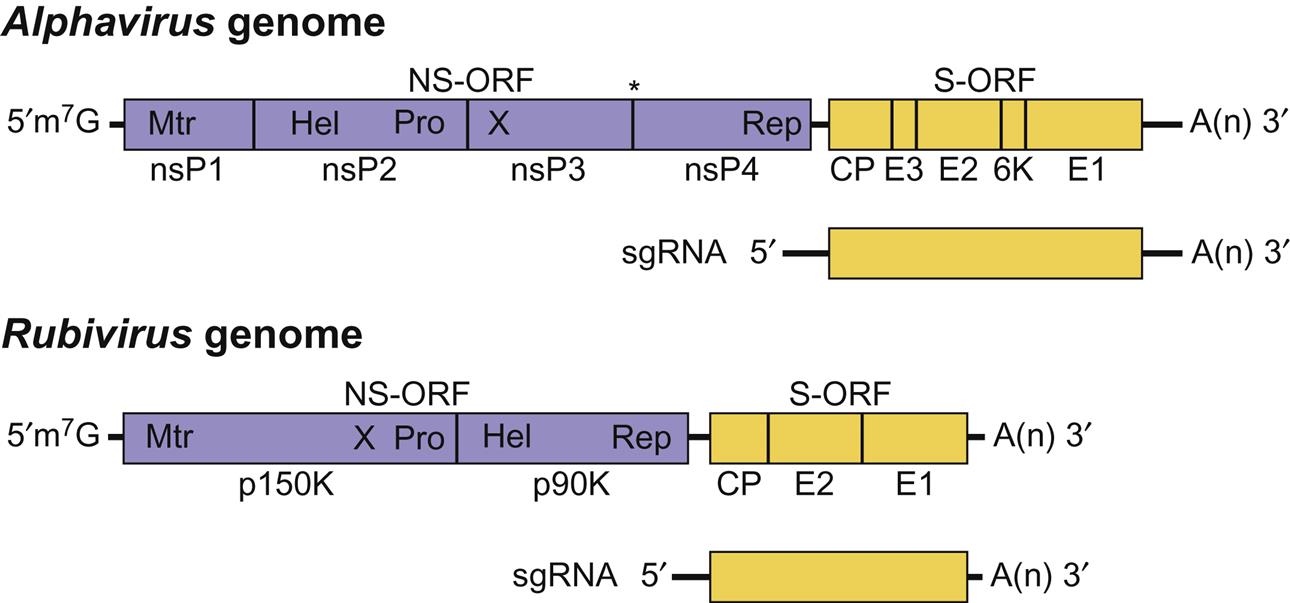
The genome is a single molecule of linear, positive-sense, single-stranded RNA, 11–12 kb in size (Fig. 28.3). The RNA has a 5′-methylated nucleotide cap and its 3′ end is polyadenylated. The 5′ two-thirds of the genome encodes nonstructural proteins; the 3′ one-third is not translated from genomic RNA, but is expressed from a subgenomic mRNA (26S) molecule that is transcribed from a full-length, negative-sense intermediate. The 26S subgenomic mRNA encodes five proteins (C, E3, E2, 6K, and E1), including a nucleocapsid protein (C, Mr 30–33 kDa) and two envelope glycoproteins (E1 and E2, Mr 45–58 kDa). Some alphaviruses have a third envelope protein, E3 (Mr 10 kDa) that is a cleavage product of a precursor protein, PE2. Alphaviruses are relatively unstable in the environment, and are inactivated by common disinfectants.
Virus Replication
Alphaviruses replicate to high titer and cause profound cytopathic changes in many kinds of vertebrate cell cultures, including: Vero (African green monkey kidney), BHK-21 (baby hamster kidney), and primary chick and duck embryo cells. They also grow in, but do not cause cytopathic changes in, mosquito cells, such as C6/36, which are derived from Aedes albopictus. In mammalian and avian cells, infection causes a complete shutdown of host-cell protein and nucleic acid synthesis. In mosquito cells there is no equivalent shutdown of these processes and cell division can continue, with the cells becoming persistently infected and continuously shedding virus.
Viral attachment to the host cell first involves interaction between the viral E2 glycoprotein and receptors on the cell surface. The broad host range of alphaviruses suggests that either E2 contains several receptor binding sites or the cell receptor is ubiquitous. Various lectins, integrins, and laminin have been identified as putative cellular receptors of individual alphaviruses. Once virions bind to the cell, the virus–receptor complex is endocytosed into coated vesicles using the clathrin-dependent pathway. Acidification of the vesicles causes a rearrangement of the E1–E2 dimer with the formation of an E1 trimer inducing fusion with the vesicle membrane with release of the nucleocapsid into the cytoplasm. Upon entry into the cytoplasm, the virion RNA has two main functions (Fig. 28.3). The 5′ end of the genomic RNA serves as messenger RNA (mRNA) that, in some alphaviruses, is first translated to produce two polyproteins, the larger of which is produced by a read-through of a weak stop codon. These nonstructural proteins in their uncleaved and cleaved forms direct the synthesis of the template negative-sense RNA genome from the input virion RNA and then genomic-size plus-strand RNA, along with a subgenomic RNA. The full-length plus-strand RNA is encapsidated into new virions, whereas the subgenomic RNA acts as message for the synthesis of the structural viral proteins. The structural proteins are expressed from the subgenomic RNA as a polyprotein that is then cleaved to form the individual proteins. In mammalian cells, nucleocapsids are assembled in the cytoplasm and move to the plasma membrane where they align under patches containing viral glycoprotein spikes. Finally, virions are formed by budding of nucleocapsids through patches of plasma membrane that are studded with spike glycoprotein (Table 28.1). The budding process in insect cells may be localized to internal cellular membranes.
New World (eg, eastern and Venezuelan equine encephalitis viruses) and Old World (eg, Sindbis, Semliki Forest viruses) apparently utilize different mechanisms to interfere with the host interferon response, which is key to their survival in infected animals. In Venezuelan and eastern equine encephalitis viruses the nucleocapsid protein, C, inhibits RNA transcription, whereas for Sindbis virus, the multifunctional nonstructural protein, nsP2, inhibits host-cell transcription. Host-cell macromolecular synthesis, including innate immune responses (see Chapter 4: Antiviral Immunity and Virus Vaccines), is compromised by both mechanisms, thus enhancing the yield of infectious virus.
Members of the Genus Alphavirus
Equine ALPHAVIRUSES (EASTERN, WESTERN and VENEZUELAN EQUINE ENCEPHALITIS)
Several closely related, mosquito-transmitted alphaviruses endemic in the Americas cause severe disease in horses and humans, and sometimes in other animal species. The most important of these are eastern, western, and Venezuelan equine encephalitis viruses. In addition to being closely related, these viruses share similar primary transmission cycles that involve mosquitoes and either birds or mammals as reservoir hosts.
The first documented outbreak of apparent alphavirus-induced encephalitis occurred among horses in Massachusetts in 1831, although the likely causative agent, eastern equine encephalitis virus, was not isolated until 1933. Incidents and epizootics of encephalitis in horses caused by eastern equine encephalitis virus have since been described throughout much of the United States east of the Mississippi River, in addition to some mid-western states and the Canadian provinces of Quebec and Ontario. Eastern equine encephalitis still occurs with regularity among horses in the eastern United States. Western equine encephalitis virus, the cause of a similar neurologic disease of horses, was first isolated from the brain of a horse in the San Joaquin Valley of California in 1931. The central role of Culex mosquitoes in the virus transmission cycle was quickly recognized, as well as the risk to humans. Extensive outbreaks of western equine encephalitis occurred throughout western North America until the middle of the 20th century, when the incidence of western equine encephalitis in horses declined precipitously and has not recurred in recent years. In 1936, an epizootic of equine encephalitis occurred in Venezuela; the causative virus was not neutralized by antibodies to the two known viruses causing encephalitis in horses elsewhere in the Americas, and was named “Venezuelan equine encephalitis virus.” These three equine encephalitis viruses initially were referred to as arboviruses because of their transmission by arthropods, and their further initial designation as “group A arboviruses” ultimately led to their current designation as alphaviruses.
Clinical Features and Epidemiology
Infection of horses with eastern, western, or Venezuelan equine encephalitis viruses produces a range of clinical manifestations that reflect the virulence of the infecting virus strain. Although the geographic range and epidemiological features of these three viruses are quite different, infections of horses can produce similar syndromes of neurological disease. These viruses are all zoonoses, and may infect other animal species, sometimes causing disease.
Encephalitic alphavirus infections of horses may be subclinical with only a transient fever, or may present with protracted fever, anorexia, tachycardia, and depression. Progressive systemic disease leading to death only occurs when the virus gains access to the central nervous system. After an incubation period of 4–6 days, affected horses develop high fever and signs of drowsiness and incoordination. The disease progresses rapidly to profound depression, typically with neurologic manifestations such an abnormally wide stance, hanging head, drooping ears, flaccid lips, irregular gait, wandering, and signs of encephalitis such as impaired vision, photophobia, inability to swallow, other reflex impairments, circling, yawning, and grinding of teeth. Constant head pressing against a corner of the stall or fence is a typical presentation. In terminal stages of disease, there is an inability to rise, paralysis, and, occasionally, convulsions. In horses, the case-fatality rate is high for North American (lineage I) eastern equine encephalitis virus infection (typically 50–90%), lower for western equine encephalitis virus infection (0–40%), and highly variable for Venezuelan equine encephalitis virus (epizootic types) infection (up to 80%). Mildly affected animals can recover slowly in a few weeks, but may have neurologic sequelae (dullness, dementia); such surviving horses have been referred to as “dummies.”
Birds are critical to the enzootic (sylvatic) transmission cycles of eastern and western encephalitis viruses. Transmission of these two alphavirus to birds is primarily through mosquito bites, but, in pheasants, eastern equine encephalitis virus has been transmitted through feather picking and cannibalism. Rodents and mosquitos are important to the enzootic transmission cycle of Venezuelan equine encephalitis virus infection.
Although the clinical signs of the equine alphavirus encephalitides are similar, the distribution and epidemiology of each is quite different.
Eastern Equine Encephalitis Virus
Eastern equine encephalitis virus is enzootic in eastern portions of North America, the Caribbean Basin, Central America, and parts of South America. Eastern equine encephalitis virus is genetically heterogeneous, and there are at least four distinct lineages (I, IIA, III, and IV) based on their antigenicity and distribution in various geographic regions. The closely related North American eastern equine encephalitis virus lineage I viruses that occur in the United States, Canada, and the Caribbean are the most virulent to humans and horses. In contrast, infection of horses or humans with the more genetically diverse virus strains enzootic in Central and South America (lineages II, III, and IV) rarely results in significant clinical disease. The lineage II strains are distributed along the coasts of South and Central America, lineage III in the Amazon Basin, and lineage IV in Brazil. These South American lineages of eastern equine encephalitis virus occasionally cause disease in horses, but rarely in people.
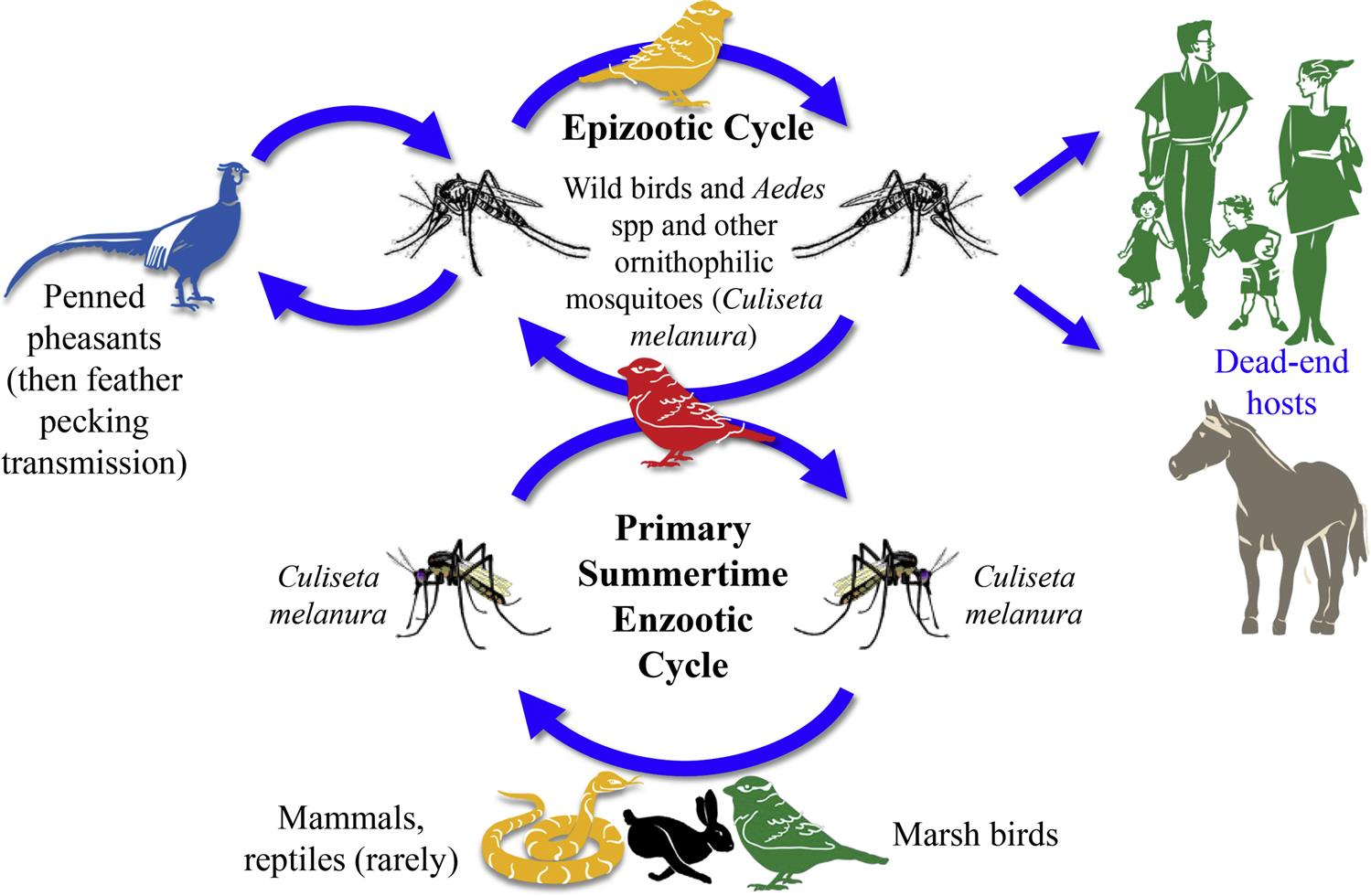
Eastern equine encephalitis virus is maintained in North America in resident birds in freshwater marshes by the ornithophilic mosquito, Culiseta melanura. This mosquito is responsible for amplification of the virus during the spring and summer by transmission among viremic wading birds, passerine songbirds, and starlings that are asymptomatic reservoir hosts of the virus (Fig. 28.4). Outbreaks of encephalitis occur in humans and horses during the late summer and fall, when the virus is acquired by other species of mosquitoes, including those in the genera Aedes and Coquillettidia that serve as “bridge” vectors that transmit the virus from its enzootic infection cycle to humans and horses. Mosquito species such as Culex peccator, Cx. erraticus, and Uranotaenia sapphirina may also serve as enzootic vectors in some regions of the southeastern United States. These mosquitoes feed on reptiles and amphibians. Eastern equine encephalitis virus apparently overwinters within enzootic regions, including temperate regions such as the northeastern United States; however, the mechanism by which this is accomplished is uncertain. Recently, snakes have been proposed as an overwintering host in the enzootic transmission cycle of eastern equine encephalitis virus. There is evidence of repeated introductions of genetically distinct virus strains into the northeastern United States from other regions, including Florida.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree