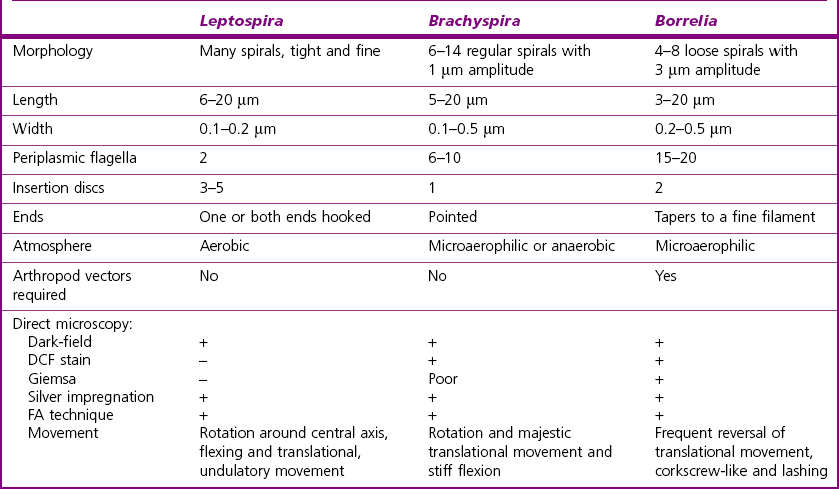
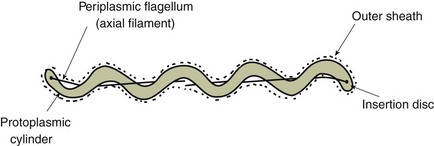
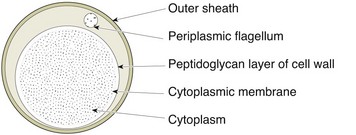
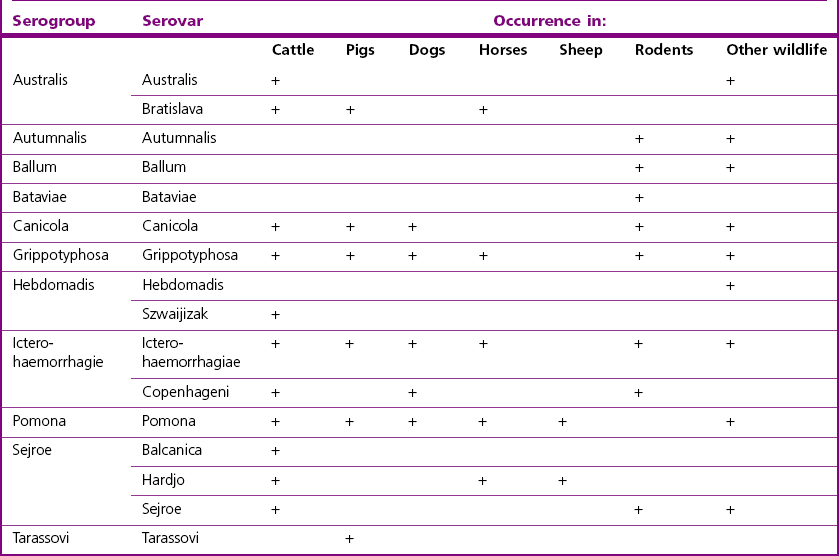
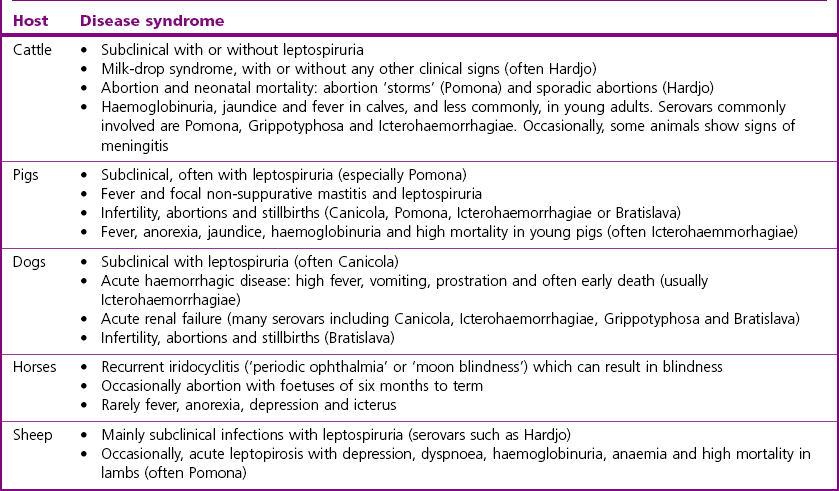
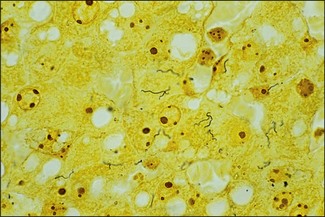
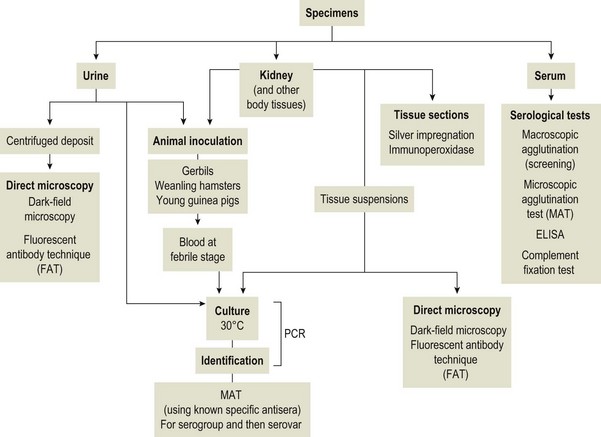
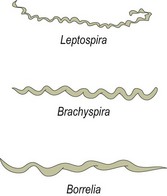
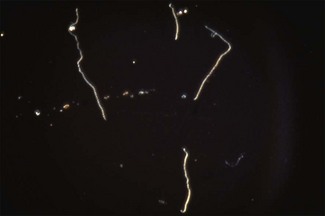
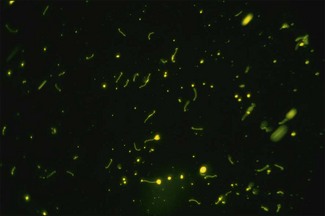
Chapter 31 The order Spirochaetales includes the families Spirochaetaceae, Brachyspiraceae and Leptospiraceae. The genera of significance in animals and humans are Leptospira (Leptospiraceae), Treponema and Borrelia (Spirochaetaceae) and Brachyspira (Brachyspiraceae). The spirochaetes are slender, motile, flexuous, unicellular, helically coiled bacteria that range from 0.1–3.0 µm in width. The outer sheath, the outermost layer of a spirochaete cell, is a multilayered membrane that completely surrounds the periplasmic endoflagella and the helical protoplasmic cylinder. The cylinder consists of the nuclear material, cytoplasm, cytoplasmic membrane and the peptidoglycan portion of the cell wall. The periplasmic flagella are wrapped around the cylinder and are in the periplasmic space of these Gram-negative bacteria. One end of each flagellum is inserted near a pole of the protoplasmic cylinder and attached by plate-like structures called insertion discs. The distal end of each flagellum is not inserted and extends to the centre of the cell and may overlap the flagellum from the opposite end (Figs. 31.1 and 31.2). The periplasmic flagella facilitate the motility of the bacteria in viscid and non-viscid environments. Table 31.1 and Figure 31.3 summarize the main differences between the genera Leptospira, Brachyspira and Borrelia, the principal genera of importance in animals. Figure 31.3 A comparison of the general morphology of Leptospira, Brachyspira and Borrelia (see also Table 31.1). The genus Leptospira was previously divided into two species, L. interrogans (parasitic) and L. biflexa (saprophytic); however, this phenotypic classification has been replaced by a genotypic one. There are currently 17 recognized genospecies including L. biflexa, L. borgpetersenii, L. interrogans and L. noguchii. Some species contain both pathogenic and non-pathogenic strains. Only non-pathogenic strains of L. biflexa have been described to date. Using the previous classification methods in which leptospires were differentiated on the basis of antigenic composition and serological reactions, more than 250 serovars in 23 serogroups were defined (Yasuda et al. 1987, Perolat et al. 1998). Serogroups and serovars do not correspond with the current genospecies and strains within the same serogroup or serovar may be found in multiple species (Table 31.2). Further taxonomic revisions of this genus are likely. Molecular classification is not yet used in clinical microbiological laboratories and until DNA-based identification methods are widely available, these laboratories are likely to retain the serological classification of pathogenic leptospires. Table 31.2 Some leptospiral serovars found in multiple species* Many serovars appear to have a certain animal species as a natural host, but animals and humans can be infected with a wide variety of serovars. The serovars causing disease in animals vary between countries and sometimes between regions in the same country. Usually the majority of infections in domestic animals, in a particular region, are caused by only a few serovars. Table 31.3 lists some of the serovars, and the genospecies and serogroups to which they belong, which have been commonly isolated from domestic and wild animals. Leptospires are present in the tubules of mammalian kidneys and are excreted in urine, often for several months. Some serovars may persist in the genital tract of carrier animals. Characteristically, a reservoir or maintenance host shows minimal or no clinical signs. A protracted shedder state is usual in maintenance hosts and they are the main source of environmental contamination. Transmission of infection can then occur to other animal species, termed incidental hosts. Incidental hosts species are less susceptible to infection than maintenance hosts but exhibit more severe disease and are inefficient transmitters of infection to other animals. Nevertheless, protacted shedding of leptospires in urine can occur in animals which have recovered from clinical disease. Significant reservoirs of animal and human leptospirosis are shown in Table 31.4. Indirect exposure depends on wet environmental conditions and a mild climate that favour the survival of leptospires. Streams and ponds can be a source of infection as can aerosols of urine in cowsheds and milk from infected cows. Leptospirosis occurs worldwide. Table 31.4 Significant reservoirs of pathogenic leptospires in animals The leptospiral genome is made up of two chromosomes, a larger one of 4400 kb and a smaller one of 350 kb in size. The full genome sequences of two serovars of Leptospira interrogans, two strains of Leptospira borgpetersenii serovar Hardjo and two strains of L. biflexa serovar Patoc have been completed (Ren et al. 2003, Nascimento et al. 2004, Picardeau et al. 2008). Comparative analysis of these genomes provides insight into the physiology and pathogenesis of pathogenic leptospires. However, many of the identified genes have no known function as it appears that the pathogenic mechanisms of leptospires differ from those of most other bacterial pathogens and homologues of these genes have not been identified in other organisms. Leptospires gain entry through mucous membranes or damaged skin as a result of direct or indirect contact. Motility and chemotaxis are essential for penetration of host tissues at this stage of infection. At least 79 motility-associated genes have been identified in the L. interrogans genome, 42 of which are common to Borrelia burgdorferi and Treponema pallidum (Nascimento et al. 2004). Colonization of host tissues is essential in the early stages of infection and afimbrial adhesins of Leptospira serovars have been characterized. After epithelial penetration there is haematogenous spread with localization and proliferation in parenchymatous organs, particularly the liver, kidneys, spleen and sometimes meninges. This phase of infection is likely to be mediated by the secretion of enzymes capable of degrading host cell membranes (Ren et al. 2003). Genes encoding both haemolysins and proteases have been identified. Some of the putative virulence factors of leptospires are given in Table 31.5. The role of some putative virulence attributes in the pathogenesis of disease has been proven through the development of mutagenesis systems for leptospires but many aspects of molecular pathogenesis remain unknown. Penetration and multiplication in the foetus can occur in pregnant animals leading to foetal death and resorption, abortion or weak offspring. The foetus, if infection occurs in the third trimester, can produce specific antibodies and may overcome the infection. Antibody production in infected animals begins a few days after the onset of leptospiraemia aand leptospires are cleared from the circulation by about 10 days after infection. Leptospires are phagocytosed by macrophages in the presence of specific antibody but virulent leptospires can induce apoptosis of macrophages both in vivo and in vitro (Mérien et al. 1997, 1998). Leptospires tend to persist in sites such as renal tubules, eyes and uterus where antibody activity is minimal. In the kidneys the organisms localize in the lumen of the proximal convoluted tubules. The relative ease with which establishment and persistence of infection occurs in maintenance hosts may be explained in part by the down regulation of some outer membrane lipoproteins in vivo and the consequent lack of recognition of infection by the immune response of the host (Haake et al. 1998). Table 31.5 Virulence attributes of pathogenic leptospires Leptospires damage vascular endothelium resulting in haemorrhages. Serovars in the serogroups Autumnalis, Grippotyphosa, Icterohaemorrhagiae and Pomona produce a haemolysin that is probably responsible for the haemoglobinuria (redwater) in young calves infected with these serovars. The pathogenesis of equine recurrent uveitis is thought to involve the production of antibodies against leptospiral antigens, probably membrane proteins, which cross-react with ocular tissues (Verma et al. 2005). Table 31.6 summarizes the syndromes of leptospirosis in domestic animals. Serological evidence indicates that a variety of leptospiral serovars can infect cats but disease appears to be uncommon in this animal. • Whole blood (serum) for serological tests. • Mid-stream urine for dark-field examination: • Mid-stream urine for attempted culture should be diluted 1 : 10 with 1% bovine serum albumin (BSA) for transport to the laboratory. Alternatively, a few drops of the urine can be aseptically added to culture media on a ‘beside-the-animal’ basis. • Whole blood (5 mL) from an animal in the leptospiraemic phase can be collected in 0.5 mL of 1.0% Na oxalate or 0.1 mL of 1.0% heparin for attempted culture of leptospires. • Foetal abomasal contents, cotyledons and uterine discharge for differential diagnosis in abortion cases. Leptospires can disintegrate quickly in a urine sample, especially if it is acidic. By adding formalin, the leptospires will be killed but will retain their morphology for several days and can be examined by dark-field microscopy. In suspected leptospirosis in cattle and pigs, the infection should be investigated on a herd basis, with urine and serum samples taken from clinically affected and in-contact animals. Leptospires may be excreted in urine intermittently and leptospiral antibody titres can vary considerably between individual animals. Figure 31.5 summarizes the specimens and laboratory tests for the diagnosis of leptospirosis. Leptospires average only 0.1–0.2 µm in width and cannot be resolved under the light microscope. They stain weakly, or not at all, with both aniline and Romanowsky stains. Leptospires can be demonstrated in urine, other body fluids and tissues by dark-field microscopy (Fig. 31.6) and fluorescent antibody (FA) technique (Fig. 31.7). Urine is centrifuged at 1500 × g for 30 minutes to concentrate the leptospires. Unclotted blood is centrifuged at low speed to sediment the red cells after which the plasma can be removed and centrifuged at 1500 × g for 30 minutes and a wet mount prepared from the sediment. Tissue specimens are ground and prepared as a 10% suspension in 1% bovine serum albumin (BSA, Fraction V powder). A drop of the preparation is placed on a microscope slide, under a cover slip, and examined by dark-field microscopy. The FA technique can be carried out on urinary deposits, tissue impression smears or frozen tissue sections. Silver impregnation or immunoperoxidase staining techniques can be used on tissue sections of kidney, liver or other tissues. Figure 31.6 Leptospira interrogans serovar Canicola from a young, actively dividing culture. (Darkfield, ×1000) Figure 31.7 Leptospira interrogans serovar Hardjo in a bovine urinary deposit. (Direct FA technique, ×400) Under dark-field microscopy leptospires can just be seen under the low power if large numbers are present. Under high-dry or oil-immersion objectives they appear as silver, beaded (due to the helical coils), slender rods with a distinct hook at each end (Fig. 31.6). Leptospiral serovars are obviously motile, if they are still viable, with flexuous movements and spinning around the long axis. The characteristic morphology can often be seen in FA or silver impregnation preparations. There is currently no accepted standard methodology for assessing the in vitro activity of antimicrobial agents against Leptospira species. Murray and Hospenthal (2004) described a broth microdilution technique for susceptibility testing which gave results within five days and employed alamarBlue as a growth detector. This group have subsequently verified that the results obtained with laboratory-adapted strains hold true for virulent organisms, lethal for hamsters (Griffith et al. 2006). Ressner et al. (2008) tested human clinical isolates from several countries worldwide and found them to be susceptible to a wide range of antimicrobial agents although there was some regional variation and the highest MICs were detected for doxycycline and tetracycline, agents traditionally used for the treatment of leptospirosis in humans.
The spirochaetes
Leptospira species
Serovar
Species
Bataviae
L. interrogans, L. santarosai
Grippotyphosa
L. interrogans, L. kirschneri
Hardjo
L. borgpetersenii, L. interrogans, L. meyeri
Icterohaemorrhagiae
L. interrogans, L. inadai
Pomona
L. interrogans, L. noguchii
Pyrogenes
L. interrogans, L. santarosai
Szwaijizak
L. interrogans, L. santarosai
Natural Habitat
Serovar
Main reservoir(s)
Alternative reservoir(s)
Autumnalis
Wildlife
Ballum
Wildlife
Bratislava
Pigs, horses, cattle
Canicola
Dogs
Cattle, pigs, rodents
Grippotyphosa
Wildlife
Cattle, pigs
Hardjo
Cattle
Sheep, deer
Icterohaemorrhagiae
Rats
Dogs, cattle, pigs
Pomona
Pigs, cattle
Dogs, wildlife
Pathogenesis
Virulence factor
Comment
Adhesion to renal epithelial cells
Adhesin not identified
Fibronectin-binding protein
Identified in virulent strains
Outer membrane proteins (Omps)
Mutation of associated gene results in attenuation
Several surface proteins, including the leptospiral immunoglobulin-like proteins
Bind host proteins and found only in pathogenic species but role in virulence unknown
Haemolysins
Sphingomyelinases identified in pathogenic species
Haem oxygenase
Utilization of haem as an iron source
Flagella
Mutation of fliY gene reduces tissue dissemination
Lipopolysaccharide
Although leptospiral LPS has reduced toxicity compared to the LPS of conventional Gram-negative pathogens, mutation and alteration of LPS biosynthesis reduces the ability of affected strains to colonize and cause disease
Laboratory Diagnosis
Specimens
 If the urine cannot be examined within 20 minutes, it should be neutralized with N/10 HCl or N/10 NaOH.
If the urine cannot be examined within 20 minutes, it should be neutralized with N/10 HCl or N/10 NaOH.
 To preserve the morphology of the leptospires for several days, 20 mL of mid-stream urine should be added immediately to 1.5 mL of 10% formalin in a 30 mL bottle.
To preserve the morphology of the leptospires for several days, 20 mL of mid-stream urine should be added immediately to 1.5 mL of 10% formalin in a 30 mL bottle.
 A small plug of kidney, taken from inside the organ with a sterile glass Pasteur pipette, can be macerated with a little distilled water and examined under dark-field microscopy.
A small plug of kidney, taken from inside the organ with a sterile glass Pasteur pipette, can be macerated with a little distilled water and examined under dark-field microscopy.
 Kidney tissue can be used for culture. Leptospires remain viable in unfrozen kidneys for several days after the death of the animal.
Kidney tissue can be used for culture. Leptospires remain viable in unfrozen kidneys for several days after the death of the animal.
 Kidney and/or liver sections in 10% formalin can be used for histopathology (Fig. 31.4).
Kidney and/or liver sections in 10% formalin can be used for histopathology (Fig. 31.4).
Direct microscopy
Antimicrobial susceptibility testing
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The spirochaetes
Only gold members can continue reading. Log In or Register to continue