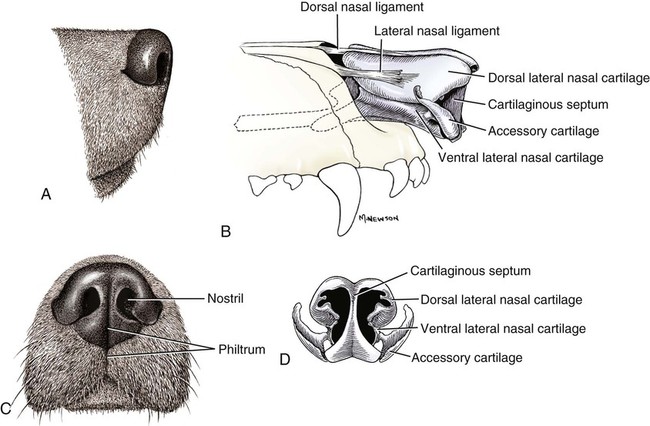
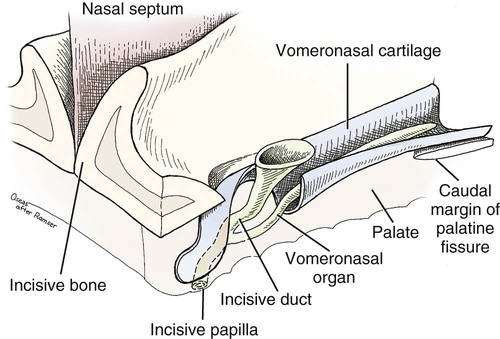
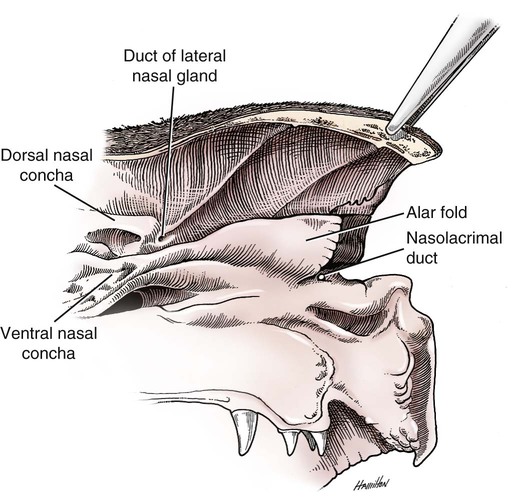
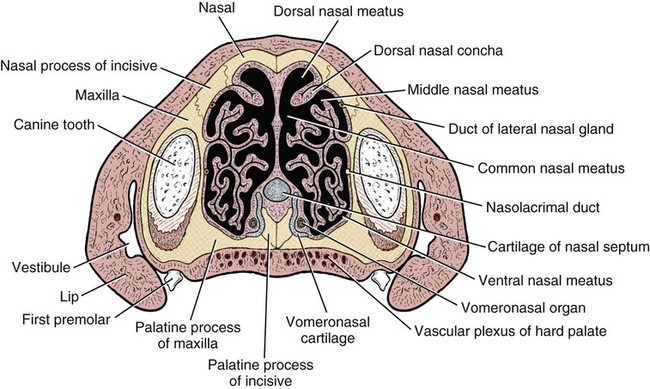
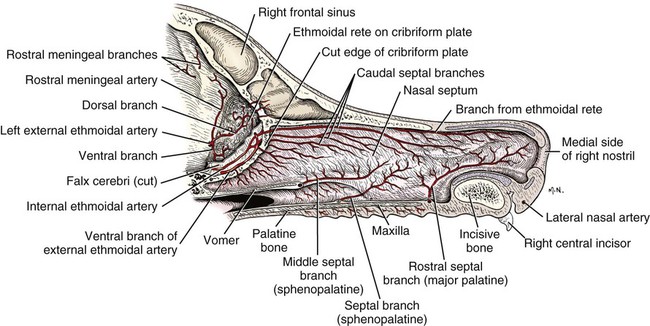
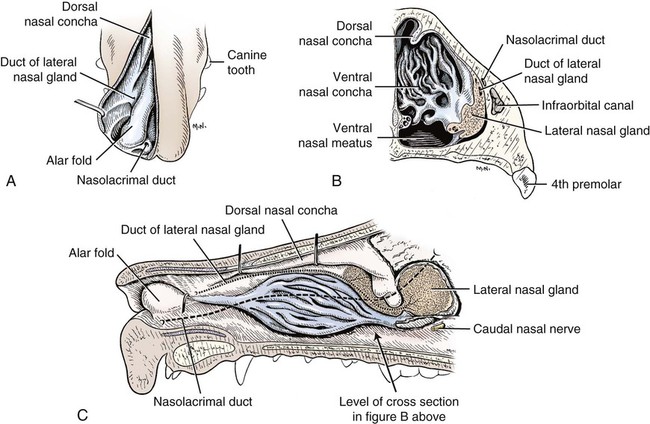
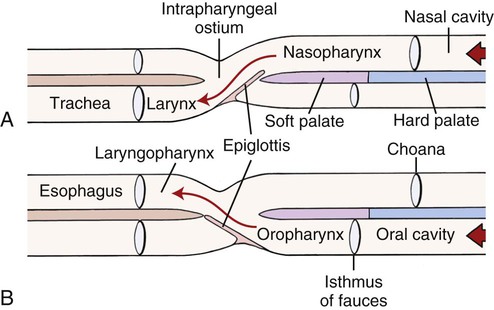
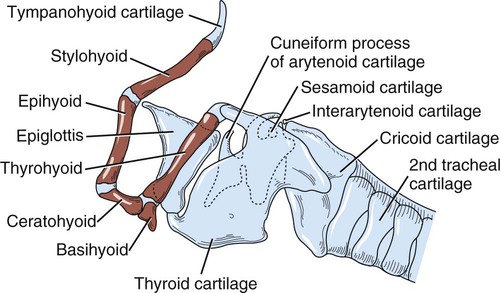
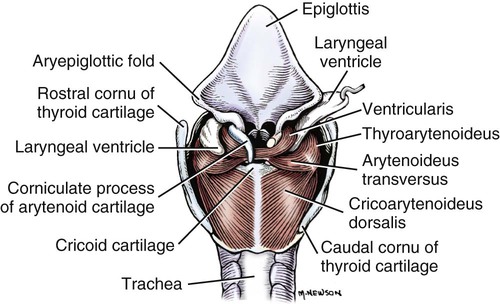
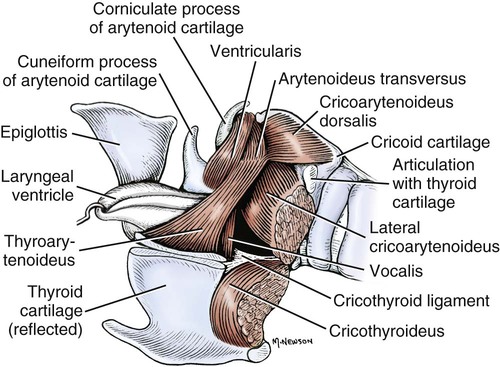
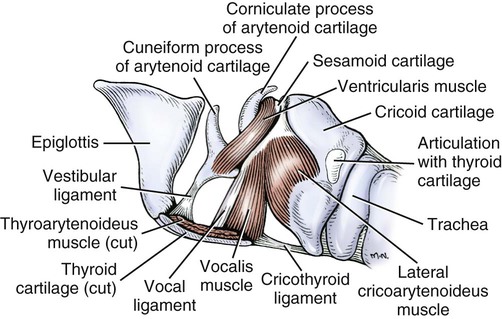
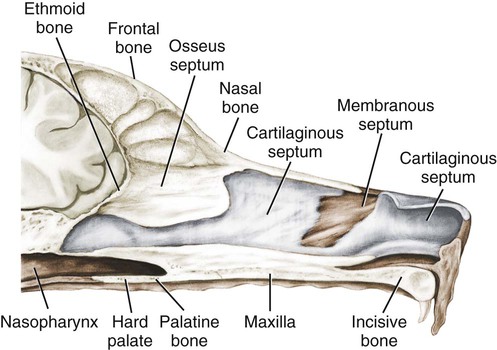
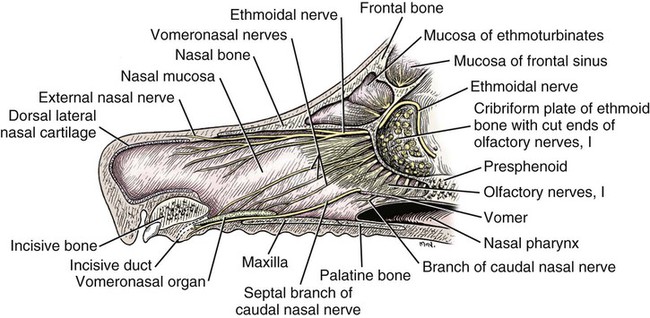
The external nose is the part of the face rostral to the frontal region and dorsal to the infraorbital, buccal, and oral regions. It consists of a fixed bony case and a cartilaginous framework. The cartilaginous portion is movable by virtue of several skeletal muscles associated with the external nose (Chapter 6). The short hair on the skin of the nose is directed caudally on the middorsal surface and gradually slopes in a caudoventral direction laterally, where it is continued on the lips. The apical portion of the nose (apex nasi) is flattened and devoid of hair. It is called the nasal plane (planum nasale) and includes the nostrils (nares), which are separated from each other by a groove, or philtrum (Fig. 8-1). The integument of the nasal plane is hairless and nonglandular. It presents epithelial elevations (areae) or papillary ridges that result in patterns characteristic for each individual. For this reason nose prints may be used as a means of identification in the dog, similar to the way fingerprints are used in humans (Horning et al., 1926). The mobile part of the external nose has a framework composed entirely of the nasal cartilages (see Fig. 8-1). These include the unpaired septal cartilage, the paired dorsal lateral and ventral lateral nasal cartilages, and the paired lateral accessory cartilages. Related to the ventral part of the septal cartilage is the vomeronasal cartilage, enclosing the organ. The rostral part of the cartilaginous nasal septum continues a median course rostrally from the membranous portion of the nasal septum (Fig. 8-2). It lies between the right and the left nasal vestibule. The rostral border of this portion of the septum is divided into right and left laminae. The cleft between the two leaves is deeper and much wider ventrally than it is dorsally. It forms a depressed triangular area ventrally. The dorsal portion of each lamina is rolled laterally to form the dorsolateral nasal cartilage. Arising from the ventral portion of the rostral part of the septal cartilage is the small ventrolateral nasal cartilage, which turns dorsally and medially toward the dorsolateral nasal cartilage (Fig. 8-1D). The accessory cartilage is united by collagenous tissue to the ventrolateral nasal cartilage. The ventrolateral nasal cartilage (cartilago nasi lateralis ventralis) (Fig. 8-1B, D) is a continuation of the rostral portion of the lateral half of the septal cartilage. Caudally, its origin moves obliquely dorsad on the lateral surface of the ventral part of the septal cartilage. It is slightly shorter and approximately one-fourth as wide as the dorsolateral cartilage. As it rolls dorsally, it is neither of uniform thickness nor of uniform curvature. Rostrally, it runs into the apex of the nose. It ends caudal to the lateral leaf of the septal cartilage adjacent to the articulation of this cartilage with the accessory cartilage. Caudally, it assumes a sigmoid shape in cross-section, being bent in such a way that its free border is added to the free border of the dorsolateral cartilage in forming the cartilaginous basis of the alar fold (plica alaris) that continues the ventral nasal concha to the vestibule. The accessory cartilage (cartilago accessoria) (see Fig. 8-1B, D) is a laterally convex leaf that articulates with the ventrolateral angle of the wide ventrally divided portion of the septal cartilage and extends dorsocaudally to the lateral surface of the expanded portion of the dorsolateral nasal cartilage. For a considerable portion of its length, it lies directly deep to the integument that covers the ventral surface of the midlateral slit in the nostril. The paired vomeronasal organ (organum vomeronasale) (Figs. 8-3, 8-6, and 8-7), long known as Jacobson’s organ, is located in the rostral base of the nasal septum as a tubular pocket of olfactory epithelium partially enclosed by a scroll of cartilage (cartilago vomeronasalis). Each vomeronasal organ opens rostrally into an incisive duct that connects the nasal and oral cavities (see Fig. 8-3). Adams and Wiekamp (1984) investigated the surface and transectional anatomy of the vomeronasal organ in the dog and described its epithelium in detail. They observed both receptor and nonreceptor areas. The receptors in the vomeronasal organ of the dog were unusual in that the cilia did not appear to be of the motile type (no dynein arms or radial spokes) and did not resemble the cilia of the cat or rabbit. Dog receptor cells did have basal bodies and apical mitochondria. The lateral wall of the vomeronasal organ has an extensive venous plexus. The neural pathways used to transmit stimuli from the vomeronasal mucosa to the brain are distinct from those of normal olfactory mucosa (Scalia & Winans, 1976). The olfactory nature of this organ in mammals was long suspected, and now we know that it plays a role in sexual behavior and kin recognition via pheromones, some of which have been identified chemically. The vomeronasal organ and its innervation was investigated by Read (1908) and Ramser (1935) in the cat and dog, McCotter (1912) in the opossum and other mammals, Mann (1961) in the bat, Kratzing (1971) in the sheep, Estes (1972) in several ungulates, and Salazar et al. (1984) in the dog. The role of olfaction in social communication in mammals was been reviewed by Eisenberg and Kleiman (1972). The effects of olfaction on the reproduction of mammals were considered by several authors in Doty (1976). Associated with the sexual role of the vomeronasal organ is the behavioral “olfactory reflex” or “lip-curl” seen in many mammals and described by Schneider (1930) as flehmen. This curling of the superior lip, which exposes the gums and teeth and allows air to be drawn in, clears the passageway to the vomeronasal organ and helps aspirate pheromones so they may contact the mucus in the vicinity of the incisive duct and be drawn into the organ to reach receptor sites. Goodwin et al. (1979) made extracts from the vaginal fluid of dogs in estrus and found that the major component of the sexual attractant was methyl p-hydroxybenzoate. The incisive duct (ductus incisivus), formerly called the nasopalatine duct or Stensen canal (Figs. 8-3 and 8-7), passes through the palatine fissure and connects the nasal and oral cavities. The oral orifice of each duct lies lateral to the incisive papilla, caudal to the superior central incisor teeth. Dogs have been observed to make rapid licking movements with the apex of the tongue, across the incisive papilla, while holding their lips slightly curled (flehmen) and their incisor teeth slightly apart. (A film of this behavior has been made by Flora Lindsey of the Glasgow Veterinary College.) The paired incisive ducts pass dorsocaudally to open into each nasal cavity, and the vomeronasal organ exits from it before it reaches the cavity. The incisive duct is not enclosed in a bony canal, nor does it contact the margin of the palatine fissure through which it passes. Three ligaments, composed of one pair and one unpaired, attach the mobile part of the nose to the dorsal portion of the osseous nose (Fig. 8-1B). The dorsal nasal ligament (lig. nasale dorsale) is a single band of collagenous tissue that runs from the caudodorsal end of dorsolateral nasal cartilage to the dorsum of the nasal bones. The lateral nasal ligament (lig. nasale laterale), one on either side, is a collagenous band that runs from the midlateral surface of the caudal aspect of the dorsolateral nasal cartilage to the border of the bony nasal aperture directly dorsal to the end of the nasomaxillary suture. The ligaments of the nose are best developed in old dogs of the working breeds. The nasal cavities (cava nasi) are the facial portion of the respiratory passageway. They extend from the nostrils (nares) to the choanae, being separated by the nasal septum. The septum (Fig. 8-2) consists of a bony portion (septum nasi osseum), a cartilaginous portion (cartilago septi nasi), and a membranous portion. Each nasal cavity has a respiratory and an olfactory region. Each nasal cavity begins at the nostril with the nasal vestibule and ends with the nasopharyngeal meatus and choana. Each nasal cavity is divided into four principal air channels and several smaller ones (Figs. 8-5 and 8-6). During development the growth of laminae from the lateral and dorsal walls of the nasal cavity results in the formation of conchae that largely fill the cavity and restrict the flow of air. The air passages thus created between the conchae are called the nasal meatuses. The nostril (naris), the opening into the nasal vestibule, is a curved opening that is much wider dorsomedially than it is ventrolaterally. It possesses more than usual importance, because in some brachycephalic dogs the opening is too restricted and interferes with respiration. Leonard (1956) devised an operation whereby the transverse diameter of the nostril may be increased. The alar fold (plica alaris) (Figs. 8-4 and 8-5), which is an extension of the ventral nasal concha, terminates within the vestibule by a bulbous enlargement that fuses to the wing of the nostril. The wing of the nostril (ala nasi) is the thickened dorsolateral portion of the nostril. The wing of the nostril contains much of the dorsolateral and accessory nasal cartilages. It is the most mobile portion of the nostril, because it receives the terminal fibers of the nasal portions of the mm. levator labii superioris and levator nasolabialis. The nasal vestibule (vestibulum nasi) is not an empty antechamber but rather it is largely obliterated by the large bulbous end of the alar fold that extends into it. Because the end of the alar fold is fused to the medial surface of the wing of the nostril, it acts to divert the incoming air. On entering the vestibule through a nostril, air is diverted medially and ventrally into the largest meatus of the nasal cavity, the ventral nasal meatus. The nasolacrimal duct (ductus nasolacrimalis), which conducts the lacrimal secretion from the eye, opens into the vestibule by an orifice located at the rostral end of the attached margin of the alar fold. The small duct of the lateral nasal gland opens on the oblique fold into the dorsal vestibule where it is continuous with the atrium of the dorsal nasal meatus. This is dorsocaudal to the opening of the nasolacrimal duct (Fig. 8-4). The dorsal nasal concha (concha nasalis dorsalis) (Figs. 8-4 and 8-5) consists of an elongated, slightly curled scroll of the first endoturbinate that is attached to the ethmoid crest (crista ethmoidalis) of the ethmoid and nasal bones. A mucosal fold continues the concha into the vestibule of the nose. The ventral nasal concha (concha nasalis ventralis) (Figs. 8-5 and 8-6) is a tightly folded series of scrolls that occupies the rostral part of the nasal cavity and is attached to the conchal crest (crista conchalis) on the medial surface of the maxilla. It extends from the level of the first to the third premolar teeth. The alar fold is an extension of this ventral concha into the vestibule. The ethmoidal conchae (conchae ethmoidales) (Fig. 8-5) form the ethmoidal labyrinth (labyrinthus ethmoidalis) that fills the caudal part of the nasal cavity. Developmentally, they are outgrowths of the ethmoid bone covered with nasal mucosa. The numerous delicate, bony scrolls are known as ethmoturbinates and are further subdivided into ectoturbinates and endoturbinates. All are attached to the orbital lamina and cribriform plate of the ethmoid. The six ectoturbinates are small and lie on the dorsal aspect of the labyrinth, whereas the four endoturbinates lie ventrally and fill the caudal portion of the nasal cavity. The first endoturbinate extends rostrally and forms the bony scroll for the concha nasalis dorsalis, and the second endoturbinate forms the middle nasal concha (Graeger, 1958). One or more scrolls of the ectoturbinates usually extend into the frontal sinus. Olfactory nerves ramify in the mucosa of the ectoturbinate scroll(s) that extends into the frontal sinus. The epithelium on the olfactory part of the sinus has a brown color. The mucosa of the nasal cavity is richly supplied with nerves and blood vessels (Figs. 8-7 and 8-8). Olfactory nerves supply approximately half of the ethmoturbinates, the caudal half of the nasal septum, and a significant portion of the roof and lateral walls of the nasal cavity. The dorsal nasal meatus (meatus nasi dorsalis) (Figs. 8-5 and 8-6) is a passage through the dorsal part of each nasal cavity. It lies between the dorsal nasal concha and the ventral surface of the nasal bone. Laterally it is limited by the ethmoidal crest, from which the concha arises. Medially the dorsal nasal meatus becomes confluent with the common nasal meatus. The common nasal meatus (meatus nasi communis) (Fig. 8-6) is a longitudinal narrow space on either side of the nasal septum. Laterally, it is bounded by the dorsal nasal concha and ventral nasal concha. Dorsal, ventral, and between these bones it is coextensive with the dorsal, ventral, and middle nasal meatuses, respectively. The paranasal sinuses, which are also connected with the respiratory passageways, are described with the skeletal system. They include a maxillary recess, a frontal sinus, and a sphenoidal sinus. The maxillary recess is not called a sinus because it is not enclosed in the maxilla. However, the opening to the recess, in life, is narrowed considerably by the mucosa-covered orbital lamina of the ethmoid bone and the lateral nasal gland located there. The frontal sinus is divided into rostral, medial, and lateral compartments. The sphenoidal sinus of the dog is only a potential cavity because it is filled by an endoturbinate scroll. Negus (1958) discusses the comparative anatomy of the nose and paranasal sinuses in various mammals. De Rycke et al. (2003) used computed tomography (CT) and magnetic resonance imaging (MRI) to provide a detailed description of the nasal cavities and paranasal sinuses in clinically normal mesaticephalic dogs. MRI scans were superior to CT scans for determining soft-tissue structures. The various types of epithelia that line the nasal cavity and coat its associated structures are spoken of collectively as the nasal mucosa. The transition from the more peripheral respiratory type of epithelium to the deeper-lying olfactory epithelium is not abrupt. The receptors for the sense of smell are located primarily on the ethmoturbinates, which lie in the caudomedial and caudodorsal parts of the nasal cavity. The olfactory epithelium and sensory nerves, so beautifully demonstrated by various techniques in several mammals by Bojsen-Møller (1964, 1967, 1975), are developed from the olfactory placode and include olfactory nerves, vomeronasal nerves, and the nervus terminalis. A richly vascularized and innervated mucosa (see Figs. 8-7 and 8-8) is present in the nasal cavity, vomeronasal organ, and in some mammals on a small area of the septum called the septal olfactory organ. Allison (1953) reviewed the olfactory system of vertebrates. Under normal conditions of inspiration, the respiratory and olfactory currents of air are associated. When a dog deliberately wants to sample the environment, the nostrils are dilated, and with a forced inspiration the dog sniffs the air. This act provides a greater volume of inspired air, which takes a more dorsal course around the ethmoturbinates, where the olfactory receptors are most numerous. Craven et al. (2007) claim that functionally the dorsal meatus is a bypass for odorant-bearing inspired air around the complicated ventral nasal concha during sniffing for olfaction. They show that within both the ventral nasal concha and ethmoturbinate air flow must be laminar. Pearsall and Verbruggen (1982) wrote a practical guide for training dogs to track scents. They include many useful behavioral observations that help delimit olfactory perception. The lateral nasal gland (glandula nasalis lateralis) (Figs. 8-6 and 8-9) is a serous gland that is located in the mucosa of the maxillary recess near the opening of this recess into the nasal cavity (Evans 1977). The lateral nasal gland was first described in the dog by Steno in 1662 and in other mammals by Bojsen-Møller (1964). The functional significance of the lateral nasal gland as part of the thermoregulatory system in the dog was postulated by Schmidt-Nielsen et al. (1970), based on airflow studies. This was confirmed by Blatt et al. (1972), who measured the amount of secretion correlated with heat-load and panting. Adams et al. (1981) described the structure of the lateral nasal gland in the dog and analyzed its secretions. The lateral nasal gland is thickest at the level of the fourth superior premolar, where its ducts unite and pass rostrally to form one major duct that opens on the lateral wall of the vestibule (Fig. 8-9). The opening of the nasolacrimal duct into the ventral vestibule is visible externally, whereas the opening of the duct of the lateral nasal gland into the dorsal vestibule is hidden from view by the alar fold (Figs. 8-4 and 8-9). The lateral nasal gland duct can be entered by a cannula with a diameter of 0.038 to 0.048 mm after surgical reflection of the dorsal wall of the vestibule. The opening lies dorsal and caudal to that of the nasolacrimal duct, where air flow is most rapid because of the narrowness of the passageway. The lamina propria of the mucosa of the respiratory part also contains serous, mucous, and mixed tubuloalveolar glands. These glands are also present in the mucosa of the nasal vestibule. Goblet cells are present throughout the respiratory region, and olfactory glands that contain yellow pigment granules are located in the olfactory epithelium. Adams and Hotchkiss (1983) described the nasal mucosa of the dog. The nasolacrimal duct (ductus nasolacrimalis) carries the serous secretion from the conjunctival sac to the nasal vestibule (Fig. 8-4). A characteristic of a healthy dog is a moist nose, which is maintained in part by the combined secretions of the lacrimal and lateral nasal glands. Much evaporative cooling in the dog is accomplished by heat exchange from the lungs and respiratory passageways. Schmidt-Nielsen et al. (1970) suggested that the lateral nasal gland supplies the water necessary for heat dissipation during panting. This function of the lateral nasal gland is in a sense analogous to that of the sweat glands of humans. Hyperventilation to get rid of excess heat can result in drying and hypertrophy of the respiratory mucosa unless moisture is provided. The nasal glands of mammals provide the necessary fluid. Blatt et al. (1972) cannulated the duct of the lateral nasal gland, subjected the dogs to various heat-loads, and collected the secretions. It was found that a rise from 25° to 40° C results in an increased flow approximately 40 times. The nasal vestibule of the dog is restricted to a residual cavity encircling the bulbous end of the alar fold, which extends into it. The alar fold is an extension of the ventral nasal concha that attaches to the wing of the nostril. Air entering the nostril is diverted dorsally, medially, and ventrally around the obstructing alar fold, with a resultant increase in velocity and evaporative effect. Perhaps the alar fold or ridges in the vestibule act as a swell body to produce cyclic alternating changes in the air passageway to protect the nasal mucosa from constant desiccation. In humans there is a cyclic distention of the cavernous tissues in the conchae and septum that results in an alternating air flow through right and left nasal chambers. Bojsen-Møller and Fahrenkrug (1971) found a similar nasal cycle in rabbits and rats. The nasal portion of the pharynx (pars nasalis pharyngis), also called the nasal pharynx or nasopharynx, extends from the choanae to the intrapharyngeal ostium. The intrapharyngeal ostium (ostium intrapharyngeum), formerly called the pharyngeal isthmus, is formed rostral to the larynx by the crossing of the digestive passageway (oral pharynx) and the respiratory passageway (nasal pharynx) (Fig. 8-10). The rostral part of the nasal pharynx is bounded by the hard palate ventrally, the vomer dorsally, and the palatine bones bilaterally. Although the middle and caudal portions of the nasopharynx are bounded dorsally by the base of the skull and the muscles that attach to it, its ventral boundary is the mobile, long soft palate (Fig. 8-11). At each act of swallowing, the cavity of the caudal part of the nasal pharynx is obliterated by the pressure of the material swallowed and the root of the tongue forcing the soft palate dorsally. On each lateral wall of the nasal pharynx, dorsal to the middle of the soft palate, is an oblique slitlike opening, approximately 5 mm long, that is the pharyngeal opening of the auditory tube (ostium pharyngeum tubale auditivae). The opening is located directly caudal to the caudal border of the pterygoid bone and faces rostroventrally. Schreider and Raabe (1981) discuss the anatomy of the nasal-pharyngeal airway of experimental animals. The larynx (Figs. 8-12 to 8-22) is a musculocartilaginous organ guarding the entrance to the trachea, which serves as an air passageway, aids vocalization, and prevents the inspiration of foreign material. The valvular function of the larynx, by means of the epiglottis, is vital, because it is across its inlet that all substances swallowed must pass in their course from the oral pharynx through the laryngeal pharynx to the esophagus. Negus (1949) has described and illustrated the comparative anatomy of the larynx from fish through mammals, and Piérard (1965) studied the dog and other carnivores. The larynx is located directly caudal to the root of the tongue, oral pharynx, and the soft palate, ventral to the atlas. It is approximately 6 cm long in a medium-sized dog, nearly half of this length being occupied by the epiglottic cartilage, which lies at the laryngeal opening. The intrinsic muscles of the larynx control the size of the laryngeal inlet, the size and shape of the glottis, and the positions of the laryngeal cartilages. Sound production with the aid of the larynx serves an important social function in canids (Tembrock, 1976). Vasquez et al. (1990) studied the normal dog larynx by MRI.
The Respiratory System
External Nose
Cartilages of the Nose
Vomeronasal Organ
Ligaments of the Nose
Nasal Cavity
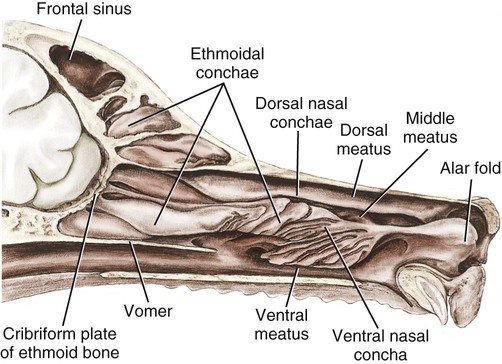
Nasal Conchae
Nasal Meatuses
Paranasal Sinuses
Nasal Mucosa
Glands of the Nose
Functional Considerations
Nasalportion of the Pharynx
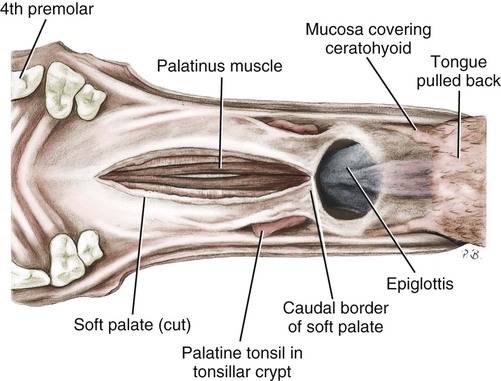
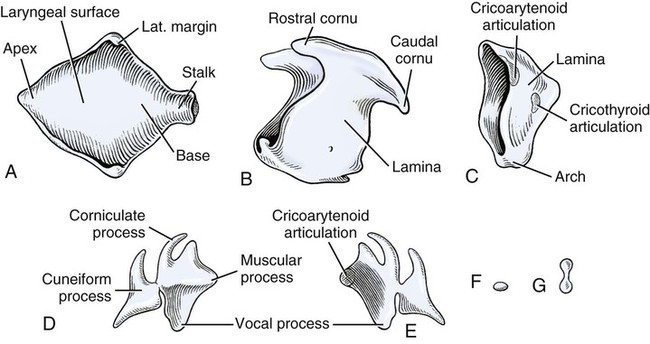
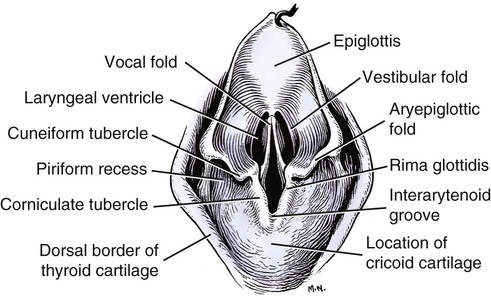
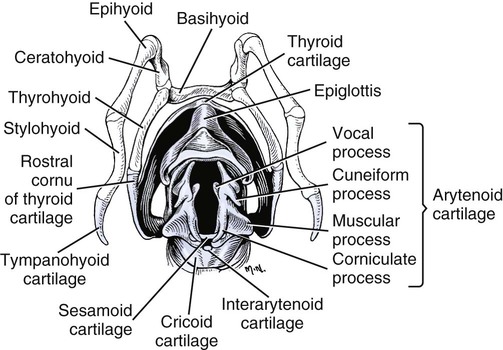
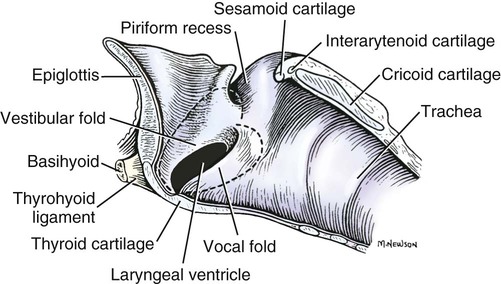
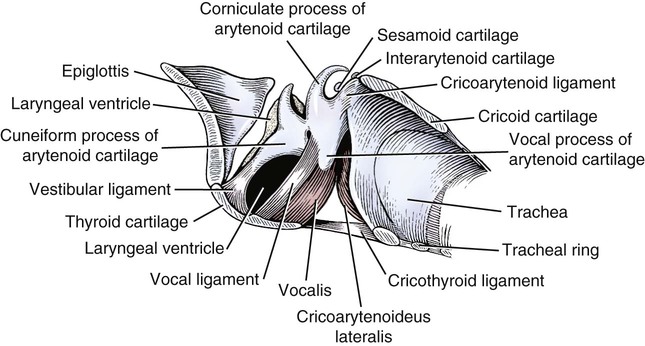
Larynx
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree