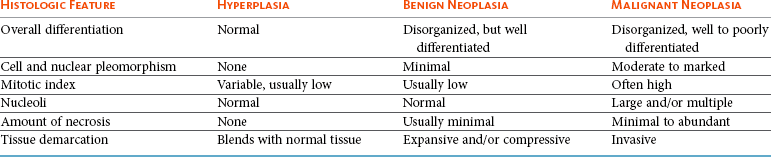
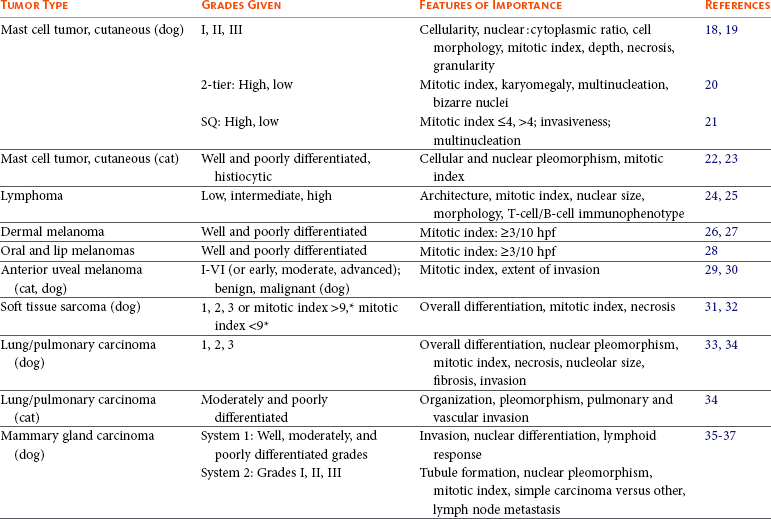
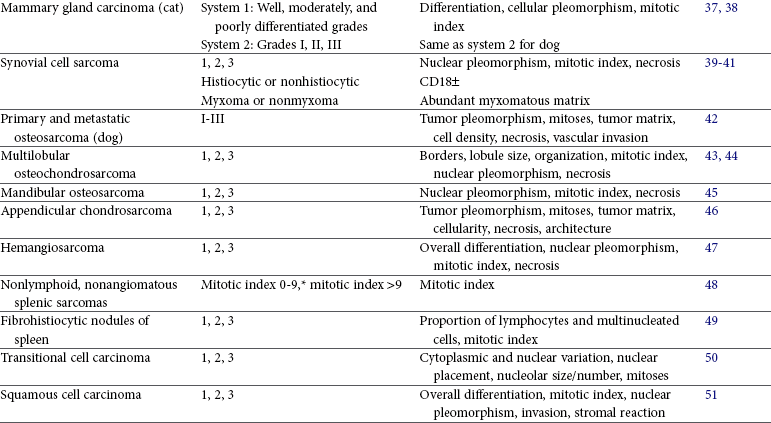
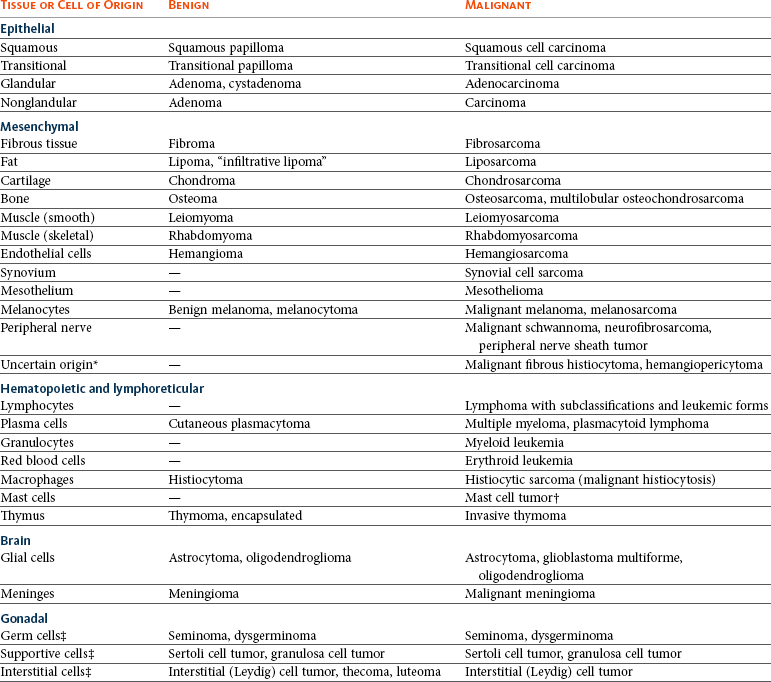
3 Veterinary pathologists play a critical role in the management of neoplasia of companion animals by providing accurate diagnostic information to clinicians so that a prognosis can be determined and adequate treatment provided. The clinician needs to have knowledge of the pathology of neoplasia to understand neoplastic conditions and the limitations of histopathologic assessment in the diagnosis of neoplasia. Both the pathologist and the clinician must work together to determine optimal treatment for the patient because the diagnosis and treatment of neoplasia in veterinary medicine have become more complex. No longer is it adequate to simply determine if the tumor is benign or malignant. The tumor type needs to be identified as accurately as possible, and tumor subtypes should be identified if prognostically significant. Grading of tumors is increasingly important because the behavior of some tumors can be predicted by the grade of the tumor. In addition, the assessment of margins for completeness of surgical removal is very important. In some cases, the histologic assessment of tissue treated preoperatively is important in predicting treatment outcome. Special procedures such as immunohistochemistry (IHC), electron microscopy (EM), flow cytometry, or polymerase chain reaction (PCR) may be advantageous in some cases to correctly identify tumor type or subtype or to predict clinical behavior of certain tumors. Classification of neoplasia in veterinary medicine is becoming more commonly applied, and systems have become more advanced with application of molecular markers for more accurate classification and prognostic information. For example, a recently upgraded histopathologic classification and grading system for canine mammary tumors has been reported to better prognostically evaluate tumors and standardize studies for cross-comparison.1 As more is learned about the diagnosis and treatment of neoplasia in veterinary medicine, newer predictive/prognostic classifications and grading schemes will continue to be developed. Very small samples can easily be lost during shipping or in processing because sample shrinkage during fixation and processing is imminent. Given these precautions, some techniques can be utilized to maximize the chances that the sample will make it through the process. Samples less than 3 mm in size can be placed on paper (surgical glove paper is appropriate) before fixation. These samples will be tacky and adhere to the paper. Very small or pale samples can be circled with pencil to draw attention to the samples at the histology laboratory. The paper can then be folded around the sample, and the entire package can be placed in formalin for fixation and shipping. Alternatively, commercially available screened tissue cassettes can be used to house the sample during fixation and shipment. The sample is placed in the screened cassette at the time of surgery, and the cassette with the sample is placed directly into formalin for fixation. These techniques decrease the chance for small samples to be lost in larger formalin containers. Extremely small samples can also be dyed with India ink or other commercially available dyes to assist in the identification of the sample. Samples containing excessive blood, mucus, or necrotic material may not be diagnostic, and the biopsy procedure may need to be repeated. If the specimen was an excisional sample, the entire sample should be submitted if feasible, and margins of concern should be identified with suture or ink. For very large samples such as large splenic tumors, representative sections such as peripheral versus central or different-colored or textured areas can be submitted. Visualization by an experienced person is extremely important when selecting the sample so that viable tissue is selected. Usually it is best to submit at least three to five sections of large lesions in case some portions of the tumor have excessive distortion, necrosis, or inflammation. When taking representative samples, the tumor and normal tissue interface should be included so assessment of tumor invasiveness into normal tissue can be determined. Tissue samples should be handled gently because compression during biopsy sampling and excessive use of electrocautery, cryosurgery, or laser surgery can cause specimen artifact, which can prevent a definitive diagnosis from being made.2 The sample needs to be preserved in fixative. The most widely used fixative is 10% neutral buffered formalin, which is readily available and frequently supplied in individual specimen containers by most laboratories. During excessive cold conditions, samples can freeze during shipment and cause significant destructive tissue artifact. Addition of 20% ethylene glycol or ethanol to the formalin can prevent freezing and maintain tissue integrity. Prior to immersion in fixative, larger samples may need to be sliced, facilitating adequate fixation; however, one side such as the deep edge should be left intact rather than slicing all the way through the sample so that orientation and margin assessment are not lost. Slices less than 1 cm thick should be avoided because curling and distortion of the tissue during fixation can result. The volume of tissue to fixative should approximate 1 : 10. In cases in which this volume ratio is not feasible because of large tumor size, multiple representative sections can be obtained. It is advisable to save the remnants of the sample, if possible, in case these first sections are not adequate for a diagnosis. When mailing large samples, use of smaller volumes of fixative is acceptable if the specimen has been in the recommended initial volume for at least 12 hours.3 Sample containers must be properly labeled (on the container, not just the lid) prior to submission to the laboratory. During transportation by mail, courier, or unpacking at the laboratory, paperwork can be inadvertently separated from the sample container, and unless the container is properly labeled, samples could become mixed. Most important, adequate history, including signalment, pertinent clinical findings, radiographic findings, and pertinent treatment, should be provided to the pathologist. A drawing of the sample indicating the position of the tissue on the animal is helpful in some situations, especially when margin determination is needed. If margins or areas of special clinical interest are marked (labeled) on the sample, a clear description of these labels, margins, or areas should be present in the submission form. A proper history is crucial for accurate diagnosis; without this the pathologist can be severely handicapped. The end result might be an inaccurate diagnosis, culminating in an inaccurate prognosis and improper treatment.2,4 Although infrequent, frozen sections can be made during operative procedures to provide the surgeon with a more rapid diagnosis. Samples are quick-frozen, sectioned on a cryostat, fixed, stained, and examined within 20 to 35 minutes. This technique is often conducted during surgery to assist with intraoperative decisions and thus requires a diagnostic laboratory and facility on site. However, these tissue sections are inferior to those processed routinely into paraffin, and as a result diagnostic accuracy is not as high and is proportional to the experience of the pathologist interpreting frozen sections. Furthermore, only a few veterinary institutions provide this service. This procedure may be helpful in establishing the identity of the tissue, adequacy of surgical margins, or adequacy of the tissue for more routine processing. Sometimes a provisional diagnosis can be made or at least a distinction between benign and malignant processes can be determined. The frozen-section diagnosis is always confirmed by routine histopathologic assessment of a paraffin-embedded section, often using the same tissue sample.4 A tumor is any tissue mass or swelling and may be neoplastic or not, although this term today more typically is a generic term used to describe any neoplasm. Neoplasia is the abnormal growth of a tissue into a mass that is not responsive to normal control mechanisms and may be benign or malignant. Growth of this mass is not affected when the inciting stimulus is removed. Cancer refers to a malignant neoplasm.5 All neoplasms arise in normal tissue and thus are composed of parenchymal and stromal cells—some can also incite secondary inflammation. Their differentiation state can be assessed with histopathology and is based on the appearance of the tumor cells, their organization, and their association with the supporting stroma. Differentiation is controlled at the molecular level by gene expression. Normal reversible processes of hyperplasia (a nonneoplastic increase in the number of cells present) and atrophy (a decrease in the number of cells present) are also composed of parenchymal cells and stroma. Their retention of near-normal architecture, just as well-differentiated neoplasms retain normal architecture, can make differentiation of the two processes difficult. At times, only removal of the inciting stimulus and time can distinguish between hyperplasia and well-differentiated cancer. One definition of cancer is a proliferation of a clonal population of cells that is no longer responsive to tissue homeostatic mechanisms. Molecular techniques to determine a clonal expansion of cells by their similar DNA sequence structure could help separate these conditions or identify occult cancer prior to tissue distortion at a microscopic level. These tests are few, but more are currently being developed, specifically for use in canine and feline lymphomas.6 Metaplasia is the abnormal transformation of a differentiated tissue of one kind into a differentiated tissue of another kind and not a neoplastic condition. Metaplasia should reverse or not progress with cessation of the chronic inciting stimulus. An example is squamous metaplasia in the prostate gland, where normally columnar epithelium becomes squamous under the influence of estrogen. Metaplastic cells can be targets for carcinogenesis if continued carcinogenic promotional events occur (e.g., bronchial squamous metaplasia in human smokers). In such cases, metaplasia often progresses and acquires dysplastic changes. Dysplasia is abnormal tissue development and can be a feature of neoplasia, but it is not necessarily a neoplastic condition. Dysplasia such as epithelial dysplasia in the oral cavity can be a preneoplastic condition. Anaplasia is a loss of differentiation or atypical differentiation and is a feature of many, but not all, malignancies. Terms associated with cellular or growth features are frequently encountered in descriptions of neoplasia. Pleomorphism is the occurrence of multiple forms, shapes, and sizes of cells and nuclei (cellular and nuclear pleomorphism respectively). Anisocytosis and anisokaryosis are greater than normal variations in cell and nuclear size, respectively. Round or polygonal cell shapes are usually associated with epithelial or hematologic tumors, whereas spindle cell shapes are usually associated with mesenchymal tumors. A scirrhous or desmoplastic response is an abundant fibroblastic proliferation with collagen formation that occurs in some malignant invasive cancers. In situ refers to a malignancy, usually limited to lesions of epithelial origin, that has not yet become invasive or invaded beyond the natural confines of its basement membrane.2,4,5 For each type of tumor, specific terminology is used to denote the origin of the tumor and whether the tumor is benign or malignant (Table 3-1). In basic terms, although benign tumors can cause tissue distortion, they typically do not have a high mortality. In contrast, malignant tumors (cancer) are more destructive of tissues and will often ultimately lead to death if the patient is left untreated. There are exceptions to these rules. Tumors can develop from any normal tissue type; therefore there are a considerable number of different tumor types. As more is learned about certain tumors, names and subclassifications may change, creating some confusion. More recent advances in molecular techniques applied to tumors have allowed additional subclassification of tumors beyond the histologic realm. More focused techniques such as IHC, targeted PCR for genetic alterations and mutations, and flow cytometry for cell surface markers can be applied once specific alterations in tumor types have been identified and determined to be markers in a specific tumor or tumor subtype. Broad encompassing techniques for large genomic, proteomic, and more recently metabolomic fingerprinting of tumors are used as discovery tools to scan for alterations that define subclasses of tumor types previously unidentified by more traditional methods. The identification of genetic mutations, varied cell surface receptor expression, altered signaling pathways, or altered cellular metabolic response to these genetic modifications may identify unique fingerprints for a tumor, which, combined with traditional histologic methods, might allow subclassification that is more accurate at identifying a tumor’s behavior and its response to tailored cancer treatment (see Chapter 8). Table 3-1 Nomenclature of Common Tumor Types in Veterinary Medicine *Pathologists disagree about the origin of these tumors; some feel they are a class of peripheral nerve sheath tumors or perivascular wall tumors. †Theoretically, all mast cell tumors are potentially malignant, but grade 1 mast cell tumors are clinically benign. ‡Unfortunately, the terminology of these tumors does not distinguish between benign and malignant forms. Benign tumors of epithelial origin are termed adenoma, papilloma, or epithelioma. Benign tumors of mesenchymal origin are designated by the suffix -oma after the tissue type (e.g., fibroma, osteoma). Malignant tumors of epithelial origin are termed carcinoma or adenocarcinoma if forming glands and ducts, whereas malignant tumors of mesenchymal origin are termed sarcoma. In some cases, the -oma suffix is used when the tumor is malignant, as in malignant melanoma and lymphoma. Leukemia, a malignant neoplasia of blood cells (occasionally referred to as “liquid tumors”) in hematopoietic tissues and usually in the blood, has no benign counterpart, although a leukemoid reaction is a nonmalignant condition that mimics leukemia.2,4,7 Although nomenclature for human cancer can often be applied to animal cancer, all nomenclature cannot be directly applied across species due to differences in tumor types and tumor behavior. Similarities and differences must be taken into account when considering whether nomenclature can have cross-species application. For example, direct comparison of canine mammary gland tumors to those of the human mammary classification system have identified differences that require an independent classification system.8 Despite recent advances in a number of areas of pathology, including molecular techniques, evaluation of tissue by light microscopy remains the standard technique for tumor diagnosis.3 Neoplasia has certain histologic features that distinguish it from hyperplasia or inflammation, and there are features that distinguish benign from malignant neoplasia. In some cases, these features can be difficult to observe. Definitive diagnosis of malignant versus benign versus inflammation or hyperplasia may not always be possible. In these cases, a repeat biopsy, either immediately or after a period of clinical observation, may facilitate a definitive diagnosis. When inflammation is present, the cellular features of reactive fibroblasts and reactive endothelial cells can be misleading.2,9 However, in reactive tissue with inflammation, the fibroblasts and endothelial cells usually are oriented perpendicular to one another (reactive granulation tissue) and usually a substantial amount of inflammation relative to reactive tissue is present. When granulomatous inflammation occurs, large reactive and epithelioid macrophages can be mistaken for tumor cells, but the pattern of tissue involvement and presence of other inflammatory cells helps rule out neoplasia. In some tumors, especially those with surface ulceration or necrosis (e.g., some synovial cell and soft tissue sarcomas), an extensive amount of inflammation can obscure neoplasia but is considered a secondary process. Benign tumors may be most difficult to distinguish from hyperplasia (Table 3-2) because both have a proliferation of well-differentiated cells that are easy to identify. There is a distortion or loss of normal tissue architecture in benign neoplasia, and usually the tumor grows in an expansive manner, causing compression rather than invasion of adjacent tissue. These tumors are often defined by a fibrous tumor capsule. Hyperplasia tends to retain normal tissue orientation and does not compress adjacent tissue. It often lacks a fibrous capsule. In general, if allowed to grow, benign neoplasia will attain a larger size than a hyperplastic lesion. In some instances, such as thyroid gland adenoma versus adenomatous hyperplasia of the thyroid gland in cats or sebaceous gland adenoma versus sebaceous gland hyperplasia the distinction between benign tumor and hyperplasia is not clinically important. Features that distinguish malignant from benign neoplasia include more dramatic loss of tissue organization, increased anisocytosis and anisokaryosis, increased nuclear and cellular pleomorphism, increased and variable nuclear : cytoplasmic ratio, abnormal nuclear chromatin, increased mitotic figures, increased and abnormal mitotic figures, abnormal large and/or multiple nucleoli, increased necrosis, amount and character of the supporting stroma, and invasiveness of malignant tumors (see Table 3-2). With invasion, individual cells or groups of tumor cells infiltrate extensively into surrounding tissue, may invade into vascular or lymphatic spaces, and may invoke a scirrhous or desmoplastic response characterized by an excessive fibrous reaction. A further feature of malignancy is destruction of the normal tissue or obliteration of normal tissue architecture. Evidence of lymph node or more widespread metastasis obviously distinguishes malignant from benign tumors.2,4,10 However, in certain tumors, histologic features do not correlate with behavior (e.g., canine histiocytoma and benign plasmacytoma). Both have histologic features of malignancy but are clinically benign. Histologically low-grade, yet biologically high-grade, fibrosarcomas of the canine head have histologic features of a benign condition but are clinically malignant.11 Similarly, bronchial carcinomas in cats will retain organized epithelial structures composed of well-differentiated ciliated pseudostratified columnar epithelium even at distant metastatic sites, including the digit, eye, heart, and kidneys.12 In these instances, knowledge of clinical history and tumor behavior is needed to distinguish benign from malignant neoplasia. In certain tumors, grading the degree of malignancy is predictive of biologic behavior,2,4,10 and, in the future, quite likely the behavior of more tumors will be shown to be related to histologic grade. Grading of tumors is somewhat subjective, and reproducibility between pathologists can be variable.13 Despite this limitation, in one study of 440,000 cases of cancer in humans, interobserver variation did not have a sufficient impact to alter the relationship between grade and outcome.14 A recent international study applying the World Health Organization (WHO) system of lymphoma classification to canine lymphomas demonstrated a reproducibility of 83% to 87% if the classification entities were well described and illustrated for reference by those applying the classification system. With preparative training of those using the system and careful application to well-described criteria, a high level of accuracy can be achieved in diagnosing canine lymphoma or any other disease entity.15 Features of tumors that are often evaluated to assess grade include (1) degree of differentiation, (2) mitotic index (number of mitotic figures per 10 high-power 400× fields), (3) degree of cellular or nuclear pleomorphism, (4) amount of necrosis, (5) invasiveness, (6) stromal reaction, (7) nucleolar size and number, (8) overall cellularity, and (9) lymphoid response (Table 3-3). Of these features, mitotic index, amount of necrosis, and nucleolar features are the only objective, quantifiable features that can be quantified with manual counting, computerized morphometry, or chemical quantification.2,4,10 Often, in determining a grade, individual features are scored, and then each score is added to obtain a total tumor score. The tumor scores are then separated into ranges that are associated with a tumor grade. Current grading scales are efficient, cost-effective, and involve no new technology. As the identification of molecular markers for tumor subtypes and prognostic or predictive parameters progresses and as the techniques become more time and cost efficient, yielding quicker turnaround times and easier application, they will become more routine and, likely, a valuable component of updated grading systems. Table 3-3 Molecular Features Underlying Grading Criteria The quantifiable criteria previously mentioned for tumor grading have more recently been assessed with image analysis (computerized analytical morphometry). The use of image analysis allows for a more objective, repeatable measure decreasing interobserver variation and bias. Examples of this methodology demonstrate nuclear features such as nuclear area, mean diameter, and perimeter that correlate with mast cell tumor histologic grade, which is predictive of tumor biologic aggressiveness.16,17 Currently, the routine application of computerized morphometry is not practical for routine use in diagnostic pathology due to time and effort restrictions, but it is only a matter of time before automation overcomes these limitations. The rationale behind the effectiveness of tumor grade is the indirect assessment of molecular features. Many of the histologic criteria used in grading scales probably reflect the underlying molecular mechanisms (Table 3-4). One example would be the correlation between tumor necrosis evaluated in many tumor grades and the underlying mechanism of tumor microvessel density (MVD) and factors that affect the density of these vessels. Lack of adequate tumor vessel density will result in tumor hypoxia and thus tumor necrosis. An underlying mechanism of tumor vessel density includes the vascular endothelial growth factor (VEGF) signaling pathway. Studies have demonstrated the strong association of MVD and VEGF expression with tumor grade and biologic tumor aggressiveness.52,53 Another example involves the induction of hypoxia-inducible factor-1α (HIF-1α) by hypoxic tumor cells. The HIF-1 gene product is the alpha subunit of transcription factor HIF-1 (http://www.ncbi.nlm.nih.gov/gene/3091). HIF-1α is a regulator of the cellular response to hypoxia by activating transcription of many genes involved in energy metabolism, angiogenesis, apoptosis, and other genes whose protein products increase oxygen delivery or facilitate metabolic adaptation to hypoxia. HIF-1α thus plays an essential role in angiogenesis and pathophysiology in the hypoxic environment present in many rapidly growing tumors. HIF-1α overexpression in brain, breast, cervical, esophageal, oropharyngeal, and ovarian cancers is correlated with treatment failure and mortality, as well as tumor progression.54 Tumor grade may correlate with survival, metastatic rate, disease-free interval, or with frequency and/or speed of local recurrence. Tumors in which grade or histologic features have been determined to be prognostic for biologic behavior in dogs include mast cell tumor18–21; lymphoma24,25; dermal, oral, and ocular melanoma26,29,54,55; mammary gland carcinoma1,35; synovial cell sarcoma39,40; multilobular osteochondrosarcoma43,44; hemangiosarcoma47; nonhematogenous sarcoma and fibrohistiocytic nodules of the spleen48,49; transitional cell carcinoma of the urinary bladder50; squamous cell carcinoma of the tongue51; lung carcinoma33,34; appendicular osteosarcoma42,56; mandibular osteosarcoma45; chondrosarcoma46; and soft tissue sarcoma31,32,57 (see Table 3-3). In humans and dogs with soft tissue sarcoma, the histologic grade is more important than the tumor type.2,32,58 Tumors whose grade or histologic features are predictive of biologic behavior in cats include lung carcinoma34 and mammary gland carcinoma,38 with conflicting reports regarding feline mast cell tumor and fibrosarcoma22,23,59,60 (see Table 3-4). Grading systems have not been well established for some malignant tumors, yet the pathologist can make an assessment of presumed biologic behavior based on the overall degree of tumor differentiation. In these cases, the terms well differentiated, moderately differentiated or poorly differentiated may be suggestive of a low-grade, medium-grade, and high-grade malignancy, respectively.61 This type of assessment is most commonly done for squamous cell carcinomas, some sarcomas, and carcinomas of the mammary gland, salivary gland, gastrointestinal tract, liver, exocrine pancreas, and perianal gland. Tumor grading probably will become even more widespread and important in the future, especially as novel analytical techniques and molecular tumor markers are included. Not only can prognosis be determined based on tumor grade and differentiation, but also treatment may be modified to apply more aggressive therapies to tumors of higher grade. The pathologist also may assist in staging of cancer by assessing tumor size, depth of tumor invasion, the presence of tumor in regional lymph nodes, and identification of tumor in distant sites. This information is needed to stage tumors into the T (tumor size and/or invasion), N (nodal involvement), and M (distant metastasis) system.10 Cytologic assessment of draining lymph nodes has been shown as a sensitive alternative to histopathology for lymph node metastasis needed for staging.62 For some tumors such as bladder cancer in humans, tumor staging is based largely on depth of tumor invasion into the bladder wall.2,4 This may prove to be useful in cases of bladder cancer in pets and has been shown to correlate with tumor grade in dogs.50 In both processes of tumor grading or tumor staging, these procedures are useful only if they have been shown to correlate with clinical behavior.
The Pathology of Neoplasia
Sample Handling
Terminology
Histologic Features of Neoplasia
Grading and Staging of Neoplasia
Grading Criteria
Underlying Molecular Mechanisms
Mitotic index
Cyclins, cyclin-dependent kinases (CDKs), proliferating cell nuclear antigen (PCNA), Ki67, bromodeoxyuridine (BrdUrd), labeling index (LI)/growth fraction (GF)
Percent necrosis
Inflammatory mediators, including eicosanoids (prostaglandins), cytokines (interleukin [IL], tumor necrosis factor alpha [TNF-α]), microvessel density (MVD)
Invasiveness
Matrix metalloproteinases (MMPs), plasminogen activators (PA), integrin expression, CAM (cell adhesion molecules)
Stromal reaction
Transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), MVD mediators
Nucleolar size
RNA transcriptional activity, silver staining nucleolar organizing regions (AgNORs)
Overall cellularity
Growth fraction, apoptosis factors (i.e., FasL, caspases), tumor doubling time
Inflammatory (lymphoid) response
TNF-α, interferon gamma (IFN-γ), IL-2, increased MHCII, intercellular adhesion molecule (ICAM)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree