CHAPTER 40 The Neurologic System
Nervous System Development
Clinically, one needs to have an elementary understanding of how the nervous system is formed to appreciate anatomical problems that can arise in this development process and the clinical effects on neonatal and young dogs and cats. In an embryo, the nervous system develops from the ectodermal layer that lies dorsal to the notochord. A proliferation of these cells forms the neuroectoderm, which is also known as the neural plate. With differential rates of cellular proliferation, this neural plate invaginates to form first a neural groove and then, when the dorsal edges meet and fuse, a neural tube with an internal neural canal extending the length of the neural tube (Figure 40-1). A group of cells on the dorsal lateral aspect of the neural tube separate and become the neural crest cells. These cells will become the neurons that form the autonomic and spinal or dorsal root ganglia and the lemmocytes (Schwann cells) that will myelinate the peripheral nervous system.
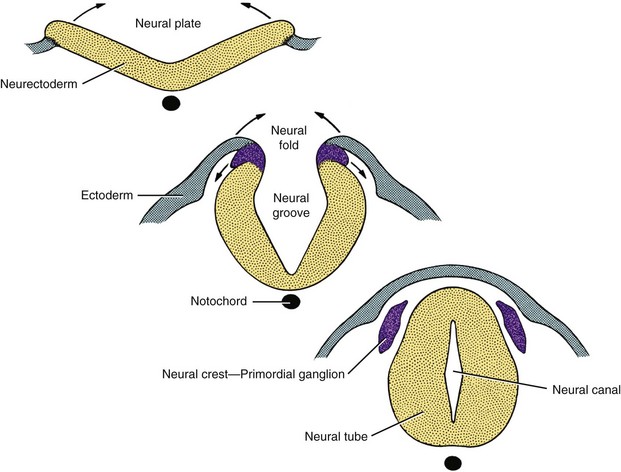
Figure 40-1 Development of the neural tube.
(From de Lahunta A, Glass E: Veterinary neuroanatomy and clinical neurology, ed 3, St Louis, 2009, Saunders/Elsevier.)
The initial fusion of the neural groove in the neural tube takes place at what will be the caudal end of the brain, the rhombencephalon, and it progresses both rostrally and caudally (Figure 40-2). For reference, scientific and common names for the subdivisions of the brain are shown in Table 40-1. The rostral section develops into the brain, and the caudal section becomes the spinal cord. The brain initially forms into three distinct vesicles: the prosencephalon, the mesencephalon, and the caudal rhombencephalon. With differential grown of neurons in these three sections, the prosencephalon further subdivides into the telencephalon, the future paired cerebral hemispheres, and the internally located diencephalon (Figure 40-3). Likewise the rhombencephalon rostrally becomes the metencephalon (pons) and caudally the myelencephalon (medulla oblongata). The cerebellum arises from rapid outgrowth of the dorsal part of the metencephalon in a structure called the rhombic lip.
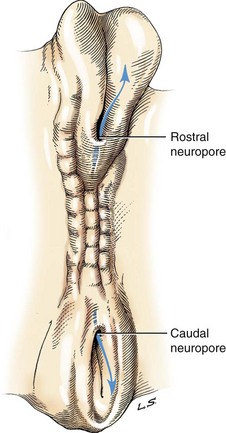
Figure 40-2 Dorsal view of neural tube closure.
(From de Lahunta A, Glass E: Veterinary neuroanatomy and clinical neurology, ed 3, St Louis, 2009, Saunders/Elsevier.)
TABLE 40-1 Scientific and common names for the subdivisions of the brain
| Scientific name | Common name |
|---|---|
| Diencephalon | No common name; contains thalamus, hypothalamus, and epithalamus |
| Mesencephalon | Midbrain |
| Metencephalon | Pons |
| Myelencephalon | Medulla oblongata |
| Telencephalon | Cerebral hemispheres; contains archicortex (hippocampus), pyriform lobe, and neocortex |
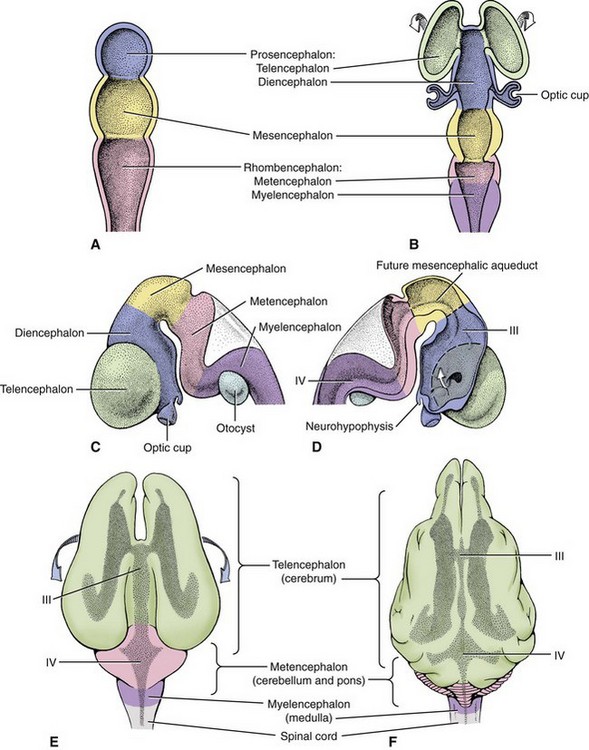
Figure 40-3 Development of brain vesicles. A, Three vesicle stages. B to F, Five vesicle stages, III, IV—ventricles.
(From de Lahunta A, Glass E: Veterinary neuroanatomy and clinical neurology, ed 3, St Louis, 2009, Saunders/Elsevier.)
Inside the developing neural tube, the space of the neural canal also changes. In the caudal part of the developing brain, the canal changes into a dorsally flattened fourth ventricle shared by the metencephalon and myelencephalon. In the mesencephalon (the midbrain), the neural canal is the least changed, remaining a narrow passageway called the mesencephalic aqueduct (cerebral aqueduct) that is subject to blockage secondary to inflammation within the brain. In the diencephalon, the canal becomes a vertically arranged space, the third ventricle, extending from the top in the epithalamus to the bottom in the infundibulum of the diencephalon. It is in the telencephalon or cerebral hemispheres where the canal is most changed as it develops from a canal into two laterally and ventrally located paired lateral ventricles that follow the massive expansion of this subdivision, the cerebral hemispheres (see Figure 40-3). Each of these lateral ventricles connects with the third ventricle through an interventricular foramen, another narrow anatomical part of the ventricular system where inflammation can cause blockage of the cerebrospinal fluid (CSF) flow. Approximately 35% of the total CSF is produced in the lateral and third ventricle by a vascular-ependymal structure called the choroid plexus. This CSF then flows caudally through the cerebral aqueduct to the fourth ventricle, where additional CSF is produced (approximately 23% of the total) and escapes from the interior of the brain through the lateral apertures in the metencephalon (pons) to flow into the subarachnoid space surrounding the brain and spinal cord. Absorption of CSF occurs primarily through one-way valves, the arachnoid granules (villi), into the venous sinuses. Malformation or inflammation that blocks the internal flow or the absorption of CSF will result in a clinical condition called hydrocephalus (see the next section).
Congenital or Anomalous Brain Conditions with Clinical Signs from Birth to 2 Months of Age
As a reference, Table 40-2 provides definitions for the congenital neurologic conditions that are described more fully in the following paragraphs. In the developing embryo, failure of the rostral segment to properly fuse and develop results in several anomalous conditions collectively called cranial dysraphism. Anencephaly occurs when the cerebral hemispheres fail to develop. This is rare and most commonly causes the fetus to die in utero, but on occasion a puppy or kitten may live to be born and then die often within hours after birth. Encephaly is a condition in which the cerebral hemispheres and meningeal coverings protrude from an opening in the calvaria. These puppies and kittens also die shortly after birth, if not before. Encephalocele is inherited in Burmese cats. In addition, pregnant queens exposed to griseofulvin may have their kittens in utero suffer these malformations and others, including agenesis of the corpus callosum and cyclopia. Reports of congenital anomalies in puppies born to bitches receiving griseofulvin during pregnancy have been reported. Meningocele, cranium bifidum, and cyclopia are also a result of failure of the rostral segment to develop correctly.
TABLE 40-2 Definition of congenital neurologic conditions
| Congenital neurologic condition | Definition |
|---|---|
| Cerebellar abiotrophy | A degenerative condition in which premature death of neurons in the cerebellum is caused by disruption of the metabolic processes necessary for cell vitality and function |
| Cerebellar hypoplasia | Failure of the cerebellar cortex to fully develop, often secondary to viral infection |
| Cerebral aplasia (anencephaly; prosencephalic hypoplasia) | Absence of cerebral hemispheres |
| Dandy-Walker syndrome | Malformation or absence of the cerebellar vermis |
| Hydranencephaly | Destruction or lack of development of the neocortex |
| Hydrocephaly | An enlargement of the cerebral ventricular system secondary to an increase in volume of CSF |
| Hydromyelia | Dilation of the central canal of the spinal cord |
| Lissencephaly | Absence of gyri and sulci in cerebral cortex |
| Meningocele | Protrusion of a fluid-filled sac of meninges through a defect in the calvaria |
| Meningoencephalocele (encephalocele) | Protrusion of brain tissue through a defect in the calvaria that is still covered by meninges and skin |
| Microencephaly | Overall reduction in the size of the brain |
| Porencephaly | Cystic cavities in the cerebral cortex usually secondary to infection |
| Syringomyelia | Cavitation of the spinal core parenchyma |
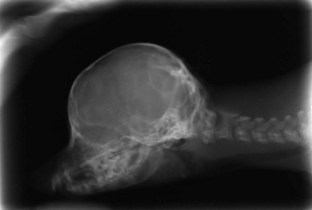
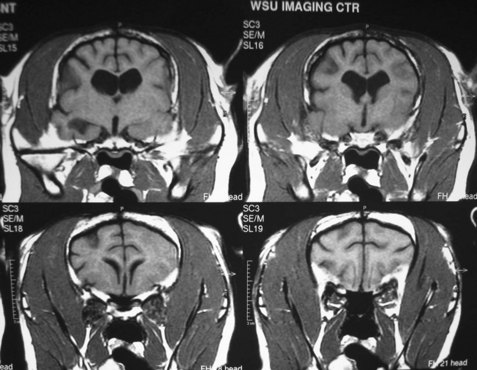
Hydrocephalus is a dilation of the ventricular system within the brain resulting in increased hydrostatic pressure from the CSF on the brain tissue, especially that adjacent to the lateral and third ventricles. This leads to either developmental failure (agenesis) or atrophy. Hydrocephalus can either be congenital or acquired after birth. Blockage of CSF flow within the brain is called internal hydrocephalus. Failure of the CSF to be absorbed, often secondary to meningitis and loss of the arachnoid granulations (arachnoid villi), is called external hydrocephalus. In either case, brain tissue fails to develop or atrophies as a result of increased CSF pressure within the skull. When this occurs in utero or shortly after birth before closure of the sutures of the skull, an enlargement of the skull occurs. The fetus may die in utero or shortly after birth. The enlarged head may cause a dystocia at the time of parturition because of the disproportionate size relative to the maternal birth canal. In less severe cases, a domed forehead may be present at birth or become evident to the owner between 2 and 3 months of age (Figure 40-4). The eyes may be malpositioned with ventral lateral deviation because of the prominent frontal areas mechanically affecting the orbits (Figure 40-5). Clinical signs of this age of puppy or kitten would include failure to thrive, seizures, behavioral signs ranging from aggression to depression, and possible absence of conscious proprioception responses and the menace response. If there are open fontanelles, ultrasound may be used to visualize the dilated ventricles. Radiographs of the skull may show an enlarged skull, thinning of the bones of the calvaria, and presence of a persisting open fontanelle (Figure 40-6). Previously the term “ground glass appearance of the calvaria” was used, which is not necessarily characteristic of hydrocephalus. Advanced imaging using computed tomography and magnetic resonance imaging (MRI) would also give a definitive diagnosis (Figure 40-7).

Figure 40-5 Ventrolateral deviation of the eyes in a 4-month-old Labrador Retriever puppy with congenital hydrocephalus.
Hydrocephalus is most commonly a congenital condition of varying degrees of severity in both dogs and cats. Small breed dogs have been reported to be at increased risk. Examples include Maltese, Chihuahua, Pekingese, Lhasa Apso, toy Poodle, Pomeranian, Pug, English Bulldog, and the small Terrier breeds. Acquired hydrocephalus is more difficult to diagnose clinically because with all skull sutures closed, the skull does not change in size. It is most commonly caused by an infectious agent affecting the brain tissue. In these cases, the clinical signs are often more severe and rapidly progressive (Figure 40-8).
Cerebellar disease is a common neurologic condition seen in young kittens and puppies. Cerebellar hypoplasia is common in kittens secondary to in utero or postnatal feline panleukopenia virus infections. The virus attacks the rapidly dividing granule cells and is cytotoxic to the Purkinje cell layer, both of which are located in the cerebellar cortex. In puppies, it is most likely an inherited condition, but canine herpesvirus and parvovirus may also be causative factors. Inherited congenital cerebellar disease is an autosomal recessive defect in Chow Chows, Bullmastiffs, Irish Setters, and some cats. It may be a primary cerebellar hypoplasia or malformation or a die-back process of a normally formed cerebellum called cerebellar abiotrophy. Table 40-3 lists the breeds of dogs and cats most commonly affected by cerebellar abiotrophy. Postnatal cerebellar abiotrophy has been reported to be an autosomal recessive inheritance in Kerry Blue Terriers, Rough-Coated Collies, and Old English Sheepdogs and is suspected in Gordon Setters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree