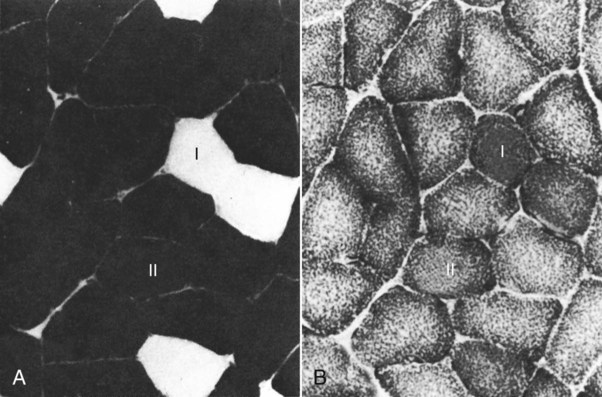
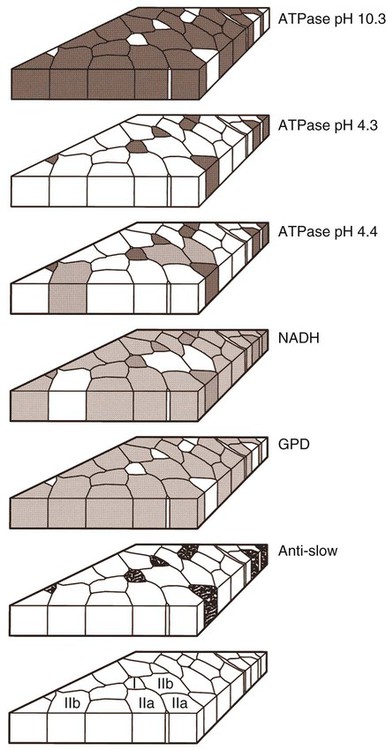
For a consideration of structural detail at the microscopic level, the reader is referred to any of the standard histology texts (Fawcett, 1986; Samuelson, 2007) and for muscle as functional units in regard to mechanics and structure see Basmajian (1974), Lieber (1992), and Biewener (1998). For overviews of biochemistry, physiology, and pharmacology see Bourne (1972, 1973), Peachey (1983), Hoyle (1983), and McMahon (1984). The phylogenetic history of muscles as seen in lower vertebrates offers many insights for explaining observed anomalies, deficiencies, or excesses in mammals (see Peters & Goslow, 1983). For a general review of comparative aspects of the muscular system in vertebrates, see Romer & Parsons (1986), Liem et al. (2000), Hildebrand and Goslow (2001), and Kardong (2008). For domestic animals, see Getty (1975) and Dyce, Sack, and Wensing (2010). At a gross level, mammalian muscle fibers are classified as red or white muscles, characteristics that are correlated with myoglobin concentration and the aerobic capacity of the muscle. Red muscle fibers (or whole muscles that are distinctly “red” in appearance) are usually specialized for repetitive or postural recruitment, contain many mitochondria, have high specific activity levels for enzymes used in aerobic metabolism such as succinic dehydrogenase (SDH), and are rich in myoglobin. Dog gastrocnemius muscles, for example, are “mixed” muscles containing slow and fast muscle fibers exhibiting high or low oxidative capacity, respectively. The medial head of m. triceps brachii is largely composed of red, aerobic, fatigue-resistant fibers (Armstrong, 1980). Good examples of “red” muscle may be better described in cats and rats in which the m. soleus (a muscle absent in dogs) is predominantly slow-twitch and highly oxidative and contrasts with the adjacent gastrocnemius muscle group. In contrast, white muscle fibers (or whole muscles with a pale “white” appearance) are involved in burst activity that requires short-duration bouts of high-force production and are exemplified by the long head of the m. triceps brachii (Armstrong, 1980). These fast and oxidative fibers are relatively fatigue resistant in dogs and facilitate the long-duration pursuits wild dogs exhibit during the pursuit of prey. These red and white muscle fiber categories have been variously described based on histochemical or immunologic studies. Two predominant systems of classification are based on either a correlation of myosin adenosine triphosphatase (ATPase) staining with metabolic properties (Peter et al., 1972) or an interpretation of myosin ATPase based on stability in acidic or alkaline buffer environments (Brooke & Kaiser, 1970; Snow et al., 1982) and myosin isoform characterization of muscle fibers (Shelton et al., 1985a; Stål et al., 1994). Controversy exists about the exclusive use of either of these systems, and it is useful to be versed in either classification (Table 6-1). Although the summary in Table 6-1 suggests some general agreement between these classifications, the two systems should not be considered interchangeable. For example, it is often assumed that the oxidative potential decreases in the order type I, IIa, and IIb. However, in rat muscles, significant overlap in either glycolytic potential or oxidative potential was found in type IIa and IIb fibers (Nemeth & Pette, 1981; Reichmann & Pette, 1982), or type IIb fibers exhibited more oxidative potential than did type IIa fibers (Reichmann & Pette, 1984). Similarly, type I fibers in rat and guinea pig were found to exhibit less oxidative potential than did type IIa fibers in the same muscle (Reichmann & Pette, 1982). Snow et al. (1982) recognize three predominant fiber types in the locomotory muscles of dogs. First, there are fibers best adapted for slow, low-force postural activity that are classified as type I fibers (Fig. 6-1). These type I fibers of dogs are comparable to type SO in the Peter et al. (1972) classification (an abbreviation referring to the slow-twitch, metabolically oxidative profiles of these fibers). These type I fibers occur in highest density in muscles active in maintaining posture, such as those active during a quiet stance (i.e., medial head of triceps brachii). A second fiber type is called type IIa and is characterized by fast-twitch, forceful contractions that are fatigue resistant. It is tempting to correlate these with the type FOG (fast-twitch, with both oxidative and glycolytic attributes that confer fatigue resistance) of other species, such as the cat (Burke, 1981). However, as stated previously, one must be cautious in applying such terminology across species. In dogs, a third fiber type occurs but is immunologically different from the type IIb fibers of cats (Fig. 6-2). Snow et al. (1982) identified these fibers as type II on the basis of antibody reactions and demonstrated that they possessed oxidative and glycolytic properties similar to those of the type IIa fibers. The type IIa fibers could be identified by their histochemical reactions and by positive immunohistochemical staining with a type IIa antibody. The presence of two populations of a “FOG-like” fiber type may represent an adaptation to the natural history of feral dogs, in which it is common to run for long distances. In correlation with muscle-specific function, varying proportions of fiber types are found in various muscles (Fig. 6-3). Recent studies in other mammals (primarily but not limited to laboratory rats) have demonstrated the presence of a novel myosin heavy chain isoform termed MHC2X (La Framboise et al., 1990; Schiaffino et al., 1989). These “IIX” (or “2X,” discussed later in this chapter) fibers are generally found in muscles that undergo repetitive contraction, such as the diaphragm of rats and mice. Based on the difficulty associated with standard histochemical separation of this fiber type population, and on the similarity of highly oxidative and glycolytic properties in these unique type II fibers, it is tempting to speculate that dog limb muscles may contain a mixture of type I, IIa, and IIx fibers. Another myosin heavy chain isoform termed MHC2D was identified by another group and named because of its occurrence in the rat diaphragm (Bär & Pette, 1988). This 2D form appears to be identical to the 2X form (La Framboise et al., 1990; Termin et al., 1989). One can see that the classification of these various fiber types has become the realm of muscle specialists. TABLE 6-1 Generalized Descriptors for and Functional Correlates of Histochemical Fiber Type Classifications Based Primarily on Analysis of Cat Triceps Surae Muscles*† FG, Fast glycolytic; FOG, Fast oxydative flycolytic; mATPase, myosin adenosine triphosphatase; SO, slow oxidative. *Data for cat medial gastrocnemius modified from Sypert and Munson (1981). Other muscles such as the cat soleus have slightly different values. Similar data summaries are not available for muscles of the dog and must account for the opinion that dogs do not have type IIb fibers in their appendicular muscles (Snow et al., 1982). †This information is provided to allow comparison of the two fiber type nomenclatures commonly used and their measured physiologic properties as generally known. 5. Medial head, triceps brachii 6. Accessory head, triceps brachii 7. Lateral head, triceps brachii 18. Humeral head, deep digital flexor 19. Radial and ulnar heads, deep digital flexor 22. Superficial digital flexor 23. Sartorius, cranial and caudal parts 42. Lateral head, gastrocnemius 43. Superficial digital flexor An additional novel fiber type has been reported in selected masticatory muscles of the dog (Mascarello et al., 1982, 1983; Shelton et al., 1985a, b) and in other carnivores (Mascarello et al., 1983; Rowlerson et al., 1981) and referred to as IIM or “superfast.” This IIM (sometimes called 2M) has been found to be immunologically differentiated from type II (IIa, IIb, or IIx) fibers of appendicular skeletal muscles of the dog. It is possible that this fiber type has evolved in those muscles associated with the first branchial arch. Reiser et al. (2009) and Toniolo et al. (2008) have shown that this masticatory-related isoform in carnivores and other mammals is, in fact, not fast-twitch, but rather is a high-force-producing muscle isoform based on analysis of isolated single myofibers in vitro. Other muscle-specific fiber types or myosin-based specializations have been reported in the extraocular muscles of mammals, which function extremely rapidly and in the absence of significant external loads (Sartore et al., 1987). For a historical review of this field of fiber types and contractile proteins see Pette and Staron (1990). An additional complication of achieving a straightforward classification of muscle fibers is the observation that some muscles may express multiple “hybrid” fibers that contain one or two myosin heavy chain isoforms (see Wu et al., 2000), and that not all research groups use identical terminology. For example, muscle types may be expressed with a Roman numeral as opposed to a digit (i.e., type IIA as compared with type 2A). For present purposes these are interchangeable. The present chapter is concerned primarily with the axial and appendicular muscles of the body. In mammalian species the skeletal muscles constitute approximately one third to one half of the total body weight. According to Gunn (1978b) Greyhounds contain the highest proportion of muscle to live weight at 57%, whereas in other dog breeds (mixed breed and purebred) this proportion is approximately 44%. Skeletal muscles range in size from the minute stapedius muscle of the middle ear to the large gluteus medius muscle of the pelvic region. Each muscle fiber is surrounded by a thin sarcolemma and a delicate connective tissue sheath known as the endomysium. When several fibers are grouped into a fasciculus they are enclosed by a connective tissue, the perimysium. The definitive muscle is composed of several fasciculi wrapped by an epimysium, which delimits one muscle from another or occasionally fuses with the intervening fascia. The size of an individual muscle fiber depends on the species, the specific muscle, as well as on the physical condition of the animal, because individual muscle fibers are capable of hypertrophy as well as atrophy. Lockhart and Brandt (1938) found muscle fibers running the entire length of the sartorius muscle (5 cm) in a human fetus, and were able to isolate fibers 34 cm long in a 52-cm sartorius muscle of an adult. Huber (1916) and Van Harreveld (1947), working with rabbit thigh muscles, found that many fibers do not extend from end to end. They concluded that, although the longer fasciculi have longer fibers, many fibers end intrafascicularly. This observation was ignored for many years until Loeb et al. (1987) demonstrated that a majority of fibers do not course the entire length of specific cat muscles. If muscle fibers exceed a certain length they become potentially inefficient because of the different conduction velocities of action potentials along nerves and muscles. Muscle fascicles appear to consist of “in-series” fibers, identified by short transverse bands of neuromuscular endplates. Similar patterns of short, “in-series” fibers have been observed in the diaphragm of dogs and cats, but not in the rabbit and rat in which fibers appear to extend from the central tendon to its costal origin (Gordon et al., 1989). Other examples of long muscles composed of short “in-series” fibers include the m. semitendinosus in the goat (Gans et al., 1989). Trotter (1990) has amplified these findings to demonstrate that tension is transmitted through “in-series” muscle fibers via the endomysium surrounding the individual fibers. Force transmission within a muscle may involve side-to-side transmission from one myofiber to another (Gao et al., 2008). Muscles take diverse shapes and are usually named according to some structural or functional feature, although other criteria have also been used. The variations encountered in the muscular system within a species are numerous and may constitute a breed-specific feature. Huntington (1903) considered problems of gross myologic research and the significance and classification of muscular variations. The most complete account of the muscles in the dog is by Baum and Zietzschmann (1936). A succinct illustrated summary of dog muscles based on the Nomina Anatomica Veterinaria (NAV) is available in Schaller (2007). For an illustrated guide to identify skeletal muscles of the dog, see Evans and de Lahunta (2009). The expanded fleshy portion of a muscle is its belly, the origin is a head. Minor divisions of origin or termination are called slips. A muscle may have more than one belly (digastric) or more than one head (triceps) and several slips. Neuromuscular compartments are regions innervated by a single primary nerve branch and separated from adjacent compartments by connective tissue partitions or epimysium (English & Letbetter, 1982a, b; English & Weeks, 1984; Galvas & Gonyea, 1980). Although functional interpretation of neuromuscular compartments is still controversial, it may provide an anatomic substrate for motor control at a higher hierarchic level than that of the motor unit (see later). Just as muscles grow and gain mass as well as strength during fetal and postnatal development, muscles show a natural aging process called sarcopenia, which includes a loss of mass and strength (Morley et al., 2001). Sarcopenia is well described in humans and in some laboratory rodents, but not well studied in dogs. Sarcopenia may be responsible for decrements of speed or performance in older dogs, and may parallel cachexia associated with disease processes. Muscles that attach to long bones (the levers) and span one or more joints usually work at a mechanical disadvantage. When a muscle fiber contracts, it does so at its maximal level of activation in an all-or-none fashion. Performance measures such as power or tension generation are complex in that they are influenced by a number of factors, such as muscle fiber length or antagonistic forces being applied against the fiber. The contraction is initiated by a nerve impulse traveling over a motor nerve fiber (axon) to the muscle fibers, or cells. Each motor neuron supplies several muscle fibers by axonal branching. These neuromuscular units are known as motor units, and the number of motor units functioning at any time determines the activity of the muscle. In general, a single motor unit corresponds with a single category of muscle fibers, such as slow- or fast-contracting fibers. The orderly recruitment of smaller and sequentially larger motor units thus correlates with the force being generated by a muscle to perform a task. Extensive reviews of motor unit physiology have been written by Burke (1981), Goslow (1985), and Stuart and Enoka (1983). If a muscle has many motor units, each of which includes only a few muscle fibers, then the precision of movement is great (as in the extrinsic muscles of the eyeball). This condition is referred to as a high innervation ratio. It is important to study and experiment with muscles in the living body to appreciate the full significance of precise muscular movement and the value of such movement in a neurologic examination for the determination of intact or defective nerve supply. Electrodiagnostic procedures are an excellent technique for studying living muscles (see Loeb & Gans, 1986). Of two muscles of equal size and shape, the muscle with the greater physiologic cross-sectional area will produce the most force (Josephson, 1975). Straplike and sheetlike muscles contract to a greater degree than do many muscles of the extremities, in part because their fibers are relatively longer and they can function efficiently over a larger range of excursions (Sacks & Roy, 1982). Examples of straplike muscles include m. gracilis and m. biceps femoris. Muscles possessing tendons throughout their length are known as pennate muscles. A muscle with a tendon running along one side is called unipennate; if there is a tendon on each side of the muscle, it is bipennate; when a muscle has tendons distributed throughout its volume, it is multipennate. Pennate muscles can be stronger because they have many short, obliquely arranged fibers and have a relatively greater physiologic cross-sectional area than similarly sized nonpennate muscles (Sacks & Roy, 1982). Examples of pennate architecture in dog muscles include m. biceps brachii and m. flexor digitorum superficialis. Because of their elasticity, tendons can protect muscles from sudden strains. However, all tendons are not constructed similarly. A range of elastic moduli can be observed in tendons obtained from different muscles in one animal. Ironically, a great deal of work performed by muscles during locomotion occurs while they are electrically active but while the muscles undergo only slight or no intrinsic length changes (Goslow et al., 1981). These eccentric contractions (being stretched while electrically active) conserve energy in several ways. First, appendicular muscles that primarily stabilize joints, such as single-joint extensors (supraspinatus, lateral head of triceps brachii), are active while the limb bears weight. By maintaining a rigid limb, the animal literally “pole-vaults” over the limbs and is able to use potential energy accrued from gravity. Second, at the higher speeds of trot and walk, these elastic storage mechanisms might be quite important as the animals store energy by stretching active muscles and subsequently recover this energy as the muscle reaccelerates the limb segment. Valuable discussions of elastic storage mechanisms are provided by Cavagna et al. (1964) and Heglund et al. (1982). The characteristic movement of a joint is produced by a muscle or muscles, called prime movers, or agonists. The muscles responsible for the opposite action are known as antagonists, although they actually aid the prime mover by relaxing in a controlled manner so that the movement will be smooth and precise. For the elbow joint, a prime mover in flexion is the brachialis; the antagonist is the triceps brachii. Conversely, in bringing about extension, the prime mover is the triceps. Fixation and articular muscles are those that stabilize joints while the prime movers are acting. Synergists are fixation muscles that stabilize intermediate or proximal joints and enable the force of the prime mover to be exerted on a more distal joint. Fascia is connective tissue that remains after the recognizable mesodermal structures have been differentiated in the fetus. It serves many important functions and has considerable clinical significance. For descriptive purposes it is convenient to distinguish many fascial entities that envelop, separate, or connect muscles, vessels, and nerves. Fascial sheets provide routes for the passage of blood vessels, lymphatics, and nerves, as well as serving for the storage of fat. Intramuscular fascial sheets also may partition neuromuscular compartments. Failure to find primary nerve branches crossing such partitions, in both neonatal and adult muscle, has been used to argue in favor of the significance of such compartments for muscle development and for functional specialization of neuromuscular compartments (Donahue & English, 1989). The superficial fascia beneath the skin is closely associated with the dermis and often includes cutaneous muscle fibers. The deep fascia that covers and passes between the muscles is particularly thick and distinct in the limbs. It functions as a sleeve within which the muscles can operate and often serves as an aponeurosis of origin or insertion. In certain locations fascia blends with the periosteum of bone, forming interosseous membranes or annular bands that confine tendons or redirect their force. Most commonly, distinct fascial septa separate groups of muscles from one another and result in fascial planes along which infection may spread or fluids drain. The amount of connective tissue present is much greater in some muscles than in others. When the connective tissue content is high, the muscle usually has many pennate fibers and thus has a high tensile strength and tends to be capable of more finely graded movements. Connective tissue elements include collagen fibers, elastic fibers, reticular fibers, fibroblasts, and histiocytes. Increased connective tissue concentrations in a muscle may also be associated with diseases, such as muscular dystrophy (Valentine et al., 1986). Muscles have a high metabolic rate and are well supplied with blood by branches from neighboring blood vessels. The arteries supplying a muscle enter at rather definite places and often anastomose within the muscle. There is much constancy in arterial supply, although variations do occur. Most dog muscles exhibit a relatively high number of capillaries per fiber compared with other mammals (Kuzon et al., 1989). This rich capillary distribution facilitates oxygen delivery to muscles necessary for endurance running performance. Lymphatics accompany the arteries and, like them, form capillary plexuses around the muscle fibers. Veins also accompany the arteries, and during muscular contraction blood is forced into the larger veins, which, as a rule, are more superficial than the arteries. Nerves accompany the blood vessels and ramify within the muscle. Approximately half of the axons in nerves are motor and the other half sensory. Efferent neurons form motor endplates, which are neuromuscular junctions on muscle fibers. Sensory receptors of a muscle include neuromuscular and neurotendinous spindles, free nerve endings, and capsulated corpuscles (Golgi tendon organs and paciniform), which discharge proprioceptive impulses in response to relaxation or contraction of the muscle, and modify the activities of motor neurons. For a summary of findings related to primary and secondary endings, static and dynamic spindles, feedback loops, and possible mechanisms of muscle spindle operation see reviews by Matthews (1972), Hunt (1990), and Hulliger (1984). Muscle spindles are not distributed evenly between or within muscles. High densities of muscle spindles have been associated with small or highly oxidative muscles (Buxton & Peck, 1990; Peck et al., 1984; Richmond & Abrahams, 1975a, b; Richmond & Bakker, 1982;). Spindles and capsulated corpuscles are apparently not present in some muscles, such as m. digastricus, in which proprioceptive feedback may be replaced by mechanical sensation from the teeth. In extraocular muscles, myotendinous cylinders or palisades are the primary receptor organs (Alvarado-Mallart & Pincon-Raymond, 1979; Richmond et al., 1984). Mammalian skeletal muscle fibers are capable of regeneration, although the success of the reparative process is variable. Regeneration results from the activation of satellite cells (myosatellitocytus) that are small cells located on the surface of striated muscle fibers. Surgical implants of minced muscle (Carlson, 1972, 1986) regenerate to approximately 25% of their former bulk, whereas transplanted whole muscle regains approximately 80% of its volume and function. The inward progression of regeneration of an implant is correlated with its revascularization. The ability of minced muscle to survive vascular deprivation is one of the striking features of muscle regeneration. The regenerative process may be aborted by conditions that stimulate connective tissue formation, such as circulatory insufficiency, widening of the gap, infection, or the presence of foreign bodies. Regulation of skeletal myocyte regeneration has been studied extensively (see reviews by Chargé & Rudnicki, 2004). The process of skeletal muscle regeneration has many applications, such as in clinical repair of injury (Carlson & Faulkner, 1983). Regeneration has been studied to assess myogenic potential during normal development or in adult animals (Ontell, 1986). Adult fiber regeneration does not recapitulate ontogeny: The number and size of myofibers in regenerating muscles are reduced relative to age-matched control subjects. Fiber type–specific deficiencies may result. Manipulation of skeletal muscles can also involve modification and transplantation for cardiac assistance (Acker et al., 1987), surgical rearrangement for orthopedic repairs (Lippincott, 1981), or reconstruction of large wounds (Miller et al., 2007). Such applications need to be performed with knowledge of the basic biologic attributes of muscle cells. The ability of cardiac muscle to regenerate is denied by some authors and supported by others. Field (1960), after reviewing the literature, concluded that, although cardiac muscle has less regenerative capacity than skeletal muscle even under optimal conditions, it does at times exhibit appreciable regeneration. More recent studies have contributed information about stem cell potential to regenerate or salvage damaged heart tissue (Balsam et al., 2004; Grounds et al., 2002; Jackson et al., 2001). Additional work examined developmental stages and inputs during myocyte ontogeny and implications for strategies to regenerate damaged heart muscle (Bu et al., 2009; Zhou et al., 2008). As a whole, the heart is complex because of the interplay of developing endothelial smooth muscle as well as intracardiac cardiomyocytes. The muscles of the head are composed of nine groups categorized primarily on the basis of their embryonic origin and their innervation (Box 6-1): (1) the muscles of facial expression innervated by the facial nerves; (2) the masticatory musculature, primarily innervated by the mandibular nerves from the trigeminal nerves; (3) the extrinsic eye musculature, innervated by the oculomotor, trochlear, and abducent nerves; (4) the tongue musculature, supplied by the hypoglossal nerves; (5) the muscles of the pharynx innervated by the glossopharyngeal and vagus nerves; (6) the soft palate muscles innervated by the trigeminal, glossopharyngeal, and vagal nerves: (7) the laryngeal musculature, supplied by the accessory and vagus nerves; (8) the hyoid muscles innervated by the trigeminal, hypoglossal, and cranial cervical nerves; and (9) cervical vertebral muscles that insert on the skull and are innervated by cervical nerves. The cranial muscles of many vertebrates have been described by Edgeworth (1935). The facial musculature of the dog has been described and illustrated by Huber (1922, 1923).
The Muscular System
Introduction
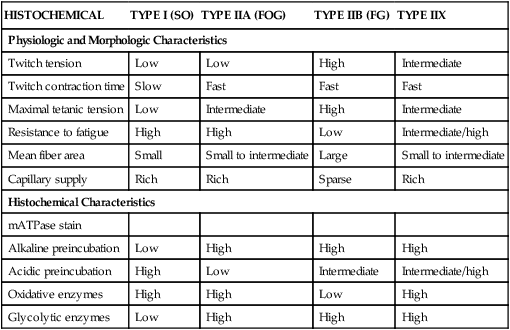
HISTOCHEMICAL
TYPE I (SO)
TYPE IIA (FOG)
TYPE IIB (FG)
TYPE IIX
Physiologic and Morphologic Characteristics
Twitch tension
Low
Low
High
Intermediate
Twitch contraction time
Slow
Fast
Fast
Fast
Maximal tetanic tension
Low
Intermediate
High
Intermediate
Resistance to fatigue
High
High
Low
Intermediate/high
Mean fiber area
Small
Small to intermediate
Large
Small to intermediate
Capillary supply
Rich
Rich
Sparse
Rich
Histochemical Characteristics
mATPase stain
Alkaline preincubation
Low
High
High
High
Acidic preincubation
High
Low
Intermediate
Intermediate/high
Oxidative enzymes
High
High
Low
High
Glycolytic enzymes
Low
High
High
High
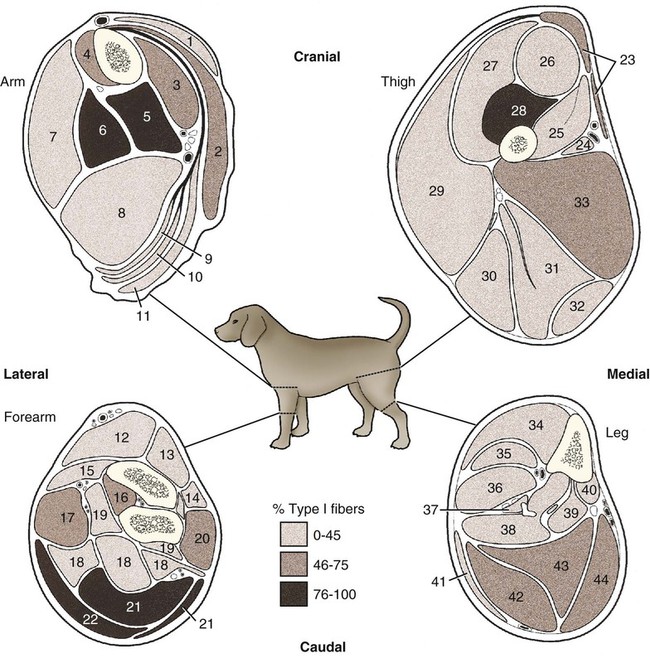
(Adapted from Armstrong RB, Saubert CW, Seeherman HJ, Taylor CR: Distribution of fiber types of locomotory muscles of dogs, Am J Anat 163:87-98, 1982. Copyright © 1982 Wiley-Liss. Reprinted with permission of John Wiley and Sons, Inc.)
Skeletal Muscles
Origin and Insertion
Function
Connective Tissue
Blood and Nerve Supply
Regeneration
Muscles of the Head