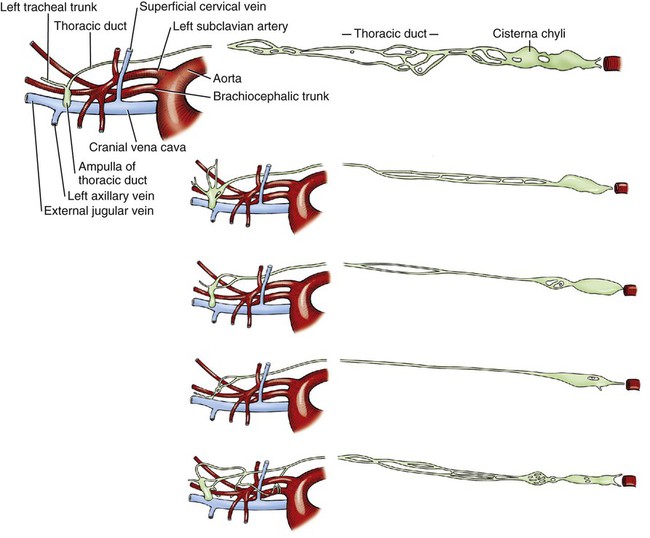
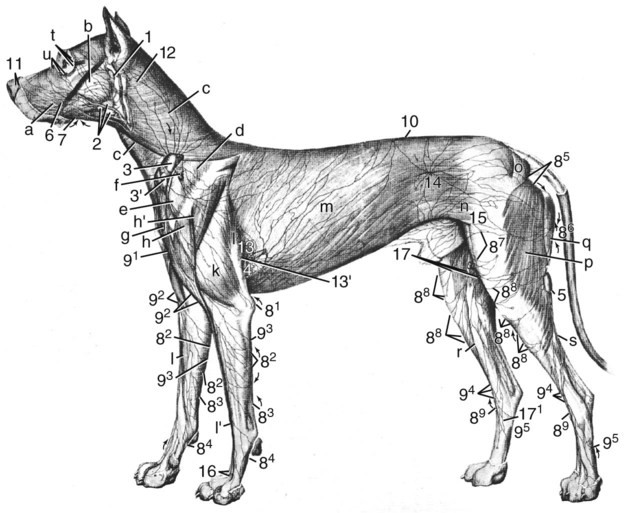
Our knowledge of the structure and function of the lymphatic system has increased substantially during recent decades. This is equally true of those aspects of the system that are of practical importance in veterinary medicine. The lymphatic system can be divided into a cellular and a vascular component. Lymphatic tissue found in all organs and lymph nodes represents the cellular component, whereas the vascular component is represented by the lymph vessel and lymph duct systems. The function of lymphatic tissue is drainage of excess tissue fluid and defense. Fixed cells found in lymph nodes, the spleen, the thymus, the tonsils, and aggregated lymph nodules are phagocytic and extract substances foreign to the body from percolating tissue fluid. Furthermore, mobile cells (lymphocytes, monocytes, and plasma cells) that circulate among lymphatic organs, blood, tissue spaces, and the lymph stream are responsible for the immune response of the body. The lymphatic vascular system includes lymph capillaries, lymph vessels, and lymph collecting ducts. For a detailed histologic description of the cells, organs, and vessels of the lymphatic system, see De Wolf-Peeters et al. (2001). Lymph from each region of the body has a characteristic composition. Garber et al. (1980) have shown that afferent renal hilar lymph contains more lymphoid cells than could be accounted for by random movement of cells from the blood to the lymph. Thus they concluded that lymphoid cells have a preferential pathway from the blood to the lymphatic system and that in the course of this journey they undergo a change that is consistent with an active immunologic role. The lymph capillaries in the villi of the intestine absorb and transport emulsified fat or chyle and therefore appear milky. They are known as lacteals. Lymph vessels of the liver (Drinker, 1946) carry proteinized lymph to the thoracic duct and, hence, to the blood, thus providing a route for stored or newly formed protein to reach the general circulation. According to Yoffey and Courtice (1956) as much as 50% of the total circulating protein escapes from the blood vessels in the course of a day. This extravascular protein and tissue fluid, which is used in part for cell nutrition, readily enters the lymphatic capillaries, along with foreign particles, if any are present. Blalock et al. (1937) attempted complete blockage of the lymph return in 52 dogs and were apparently successful in three instances. Threefoot (1968) demonstrated lymphaticovenous communications to nearly all veins of the body, but they were most common in the region of the renal veins. Zajac (1972) reported communications between the cisterna chyli and the caudal vena cava. Furthermore, Kagan and Breznock (1979) and Griaznova (1962) created a complete obstruction of the thoracic duct and found that the lymphatic system responded by opening lymphaticovenous anastomoses proximal to the site of obstruction. Anastomoses exist between lymph vessels and veins in several organs (Clark & Clark, 1937), in addition to the main connections at the base of the neck. Pina and Tavares (1980) also demonstrated that the caliber of lymph vessels in the heart increased during the first week after ligation of the superficial veins and decreased thereafter as effective collateral venous drainage was established. Lymph capillaries are simple, transparent endothelial tubes. Huntington (1908, 1910, 1914), McClure (1915), and Kampmeier (1969) reviewed the existing literature and concluded that the lymphatic system arises by confluence of perivenous mesenchymal spaces to form larger spaces, these in turn becoming confluent to form continuous vessels that eventually open into the venous system. The cells lining the spaces at first are undifferentiated mesenchyme but later become flattened to form the endothelium of the lymph vessels. The consecutive development of blood vascular endothelium and lymph vessel endothelium led to the hypothesis that lymph vessel endothelium is derived from neighboring blood vascular endothelium (Sabin, 1911). Töndury and Kubik (1972) suggested that both processes are important in the development of the lymph vessels. First, endothelial buds appear on certain parts of the venous system, and these generally lose their connections with their parent vessel. Second, the lymph vascular system can develop from endothelium-lined perivenous mesenchymal spaces that at first consist of lacunae, which then coalesce and form a secondary link with the venous system. The dual origin of the lymphatic system is supported on the basis of the expression pattern of homeobox transcription factor Prox 1 (Oliver & Harvey, 2002) and by the combination of the expression pattern of Prox 1 and grafting experiments (Wilting et al., 2006). By this means a primitive lymphovascular system develops along all major blood vessels that consist of paired jugular sacs in the neck region, a caudal sac that is made up of paired lumbar sacs and a reticulate iliac part, an inguinal sac that goes to the inguinal region, an unpaired retroperitoneal sac, and a cisterna chyli. The original connections with the veins may be retained as the definitive connections of the adult lymphatic system, or they may disappear entirely or be reestablished later. Except for the cisterna chyli, most sacs do not persist as such in the adult. In mammals the sacs ultimately become primary lymph nodes, whereas secondary nodes develop along the course of the lymph ducts (Yoffey & Courtice, 1956). The larger lymph collecting vessels are surrounded by smooth muscle and a fibrous adventitia. Although segments of the lymph vessels are contractile, the musculature is poorly organized (Threefoot, 1968). The vessels occasionally exhibit intrinsic pulsations, although the flow of lymph depends mainly on the movement of adjacent muscles. Lymph vessels are not necessarily present wherever there are veins. The pattern and distribution of the lymph capillaries and lymph vessels are determined largely by the function of the organ. Baum devoted a large part of his work on the lymphatic system to a systematic description of the lymph drainage of various tissues. For a detailed description of the lymph drainage of the various tissues and organs of the dog, see Baum (1918) (Fig. 13-1). The following is a brief summary of the lymph drainage of various tissues of the body. 2. 2′, 2″ Mandibular lymph nodes 3, 3′ Superficial cervical lymph nodes 4. Accessory axillary lymph node 6. Lymph vessels of the gums on the buccal side of the maxillary teeth 7. Lymph vessels of the gums on the buccal side of the mandibular teeth 81 to 99. Lymph vessels that course to the medial side of the limb (those numbered 85 to 88 go to the superficial inguinal lymph nodes) 91. Lymph vessels of the skin of the cranial pectoral region 92 to 95. Lymph vessels that course to the lateral side of the limb 10. Lymph vessel that crosses over the dorsal midline 11. Lymph vessels of the muzzle 12. Lymph vessel that passes deep to the medial retropharyngeal lymph node 13-13′ Lymph vessels that go to the axillary lymph node 14. Lymph vessels that go to the medial iliac lymph node 15. Lymph vessels that enter the superficial inguinal lymph nodes 16. Lymph vessels that pass from the palmar to the dorsal side of the forepaw 17-17′ Lymph vessels that go to the superficial inguinal lymph nodes Sparse networks of lymph capillaries are present in the periosteum and endosteum of bone, and lymph capillaries follow the pattern of the Haversian canals in compact bone. Bone marrow and cartilage do not have lymph capillaries or lymph vessels. Lymph capillaries are present in the endomysium between individual muscle fibers, between tendon fibers, and subsynovially in tendon sheaths and synovial bursae. Yoffey and Courtice (1956) described lymph vessels in the fascial planes between muscles. The central nervous system and the meninges do not have lymph capillaries, although lymph vessels have been described in the perivascular connective tissue of the parenchyma, the intervertebral foramina and epidural fat tissue. Földi et al. (1966) have shown that lymph vessels are present in the substance of the dura mater at the base of the skull and that these vessels have connections to the lymph vessels in the neck. Drainage of cerebrospinal fluid via lymph passages that accompany the olfactory nerves from the nasal cavity, as well as lymph vessels in the perineural and perivascular connective tissue, is of special functional significance. Serous membranes also have a very rich lymph capillary network that plays an important role in reabsorption of serous fluid from the body cavities. Lymph from the peritoneal cavity drains into the lymph vessels of the diaphragm and from there travels via five different routes (of which the ventral sternal and pulmonary routes are the most important) back to the general circulation (Higgins & Graham, 1929). Subserous and submucosal lymph plexuses are present in the digestive tract, although their arrangement is different in the various segments of the digestive tract (Durovičová & Munka, 1974; Hirashima et al., 1984; Tanigawa, 1978; Unthank & Bohlen, 1988; Womack et al., 1988). The lymph capillaries of the liver, pancreas, and salivary glands lie in the perilobular or periacinar interstitial connective tissue. They do not arise within the liver lobules or acini of the pancreas and salivary glands. Pissas (1984) described two different lymph channels draining the pancreas, one draining the left lobe and one draining the right lobe. The alveoli of the lungs do not have any lymph capillaries, but peribronchial, perivascular, and subpleural networks are present (Rodrigues-Grande et al., 1983). The lymph vessels of the lung have recently attracted much attention because of their role in the development of lung edema caused by positive end-expiratory pressure and lung damage (Bethune et al., 1979; Drake et al., 1987; Frostell et al., 1987; Haider et al., 1987; Oberdörster et al., 1978; Rodrigues-Grande et al., 1983). On average, half of lung external lymph drainage goes into the right lymph duct and thoracic duct. Furthermore, in experimentally induced pulmonary edema 70% to 100% of lung lymph enters the right lymph duct (Martin et al., 1983; Pieper et al. 1986; Vreim et al., 1977) Two sets of lymph vessels drain the dog kidney, namely, a smaller capsular and a larger hilar system. Connections between the two systems exist (Holmes et al., 1977). Although earlier workers state that there are no lymph passages in the parenchyma of the renal cortex or medulla, Nordquist et al. (1973) and Eliska (1984) confirmed that the renal cortex contains large numbers of fenestrated lymph capillaries that are distributed among arteries, veins, tubules, and renal corpuscles. Albertine and O’Morchoe (1981) describe four types of lymph vessels in the kidney of the dog. The ureter has lymph vessels in its adventitia, whereas the urinary bladder has submucosal, intermuscular, and subserosal lymph plexuses. Subendocardial, myocardial, and subepicardial lymph capillary plexuses drain the heart (Eliska & Elišková, 1980; Elišková & Eliska, 1974; Kerestanová and Slezák, 1983; Schmidtova et al., 1974; Shimada et al., 1989), and lymph from the pericardial space drains into the coronary lymph vessels (Miller et al., 1988). Eliska et al. (1999) found that the coronary arteries are drained by adventitial lymph vessels that do not penetrate to the tunica media and via periadventitial lymph vessels consisting of a subepicardial lymph plexus overlying the arteries. Smaller arterioles in the ventricular muscle have many more accompanying lymph vessels than do epicardial coronary arteries. The parietal pericardium has a complicated network of lymph vessels that is different from the lymph vessels that drain the pericardial cavity (Elišková et al. 1994). The dorsal area of the pericardium drains directly into cranial mediastinal lymph nodes; the cranial and lateral areas drain into lymph vessels that pass cranially and caudally along the phrenic nerves. Those that pass cranially drain into cranial mediastinal lymph nodes. Those that pass caudally drain to the lymph vessels from the area of the diaphragm. The large lymph vessels (vasa lymphatica) have walls thinner than those of similar sized veins but contain more valves. (For a comparison of the viscoelastic properties of the walls and functional characteristics of the valves in lymph vessels and veins, see Ohhashi [1987].) Furthermore, central and peripheral lymph vessels have subsurface and surface morphologic features distinct from either arteries or veins (Gnepp & Green, 1979). When the flow of lymph is obstructed, the vessels become distended, and, owing to constrictions at the valves, they often resemble a string of beads in appearance. (For a discussion of the structure of lymph vessels and their valves see Gnepp and Green [1980], Albertine et al. [1982], and Todd and Bernard [1974].) Blockage of lymph vessels results in an accumulation of tissue fluid and consequent swelling, known as lymphedema. Lymph vessels, when cut, remain open longer than do comparable blood vessels, but they have remarkable regenerative capacities (Danese et al., 1962; Reichert, 1926). Reichert (1926) found that the lymph vessels of the thigh of the dog regenerated rapidly after being transected. The cutaneous lymph vessels began to regenerate after 4 days, and the deep lymph vessels after 8 days. Meyer (1906), however, found no regeneration after ligating and resecting 3 to 5 mm of the large lymph trunks in the pelvic limb of the dog. Because lymph vessels contain numerous valves, they are difficult to inject in a retrograde direction. The valves are usually bicuspid, although occasional tricuspid and monocuspid valves are found (Albertine et al., 1982). They may best be observed by producing congestion after ligation, by injecting dye particles peripherally, or in edematous tissue. Prier et al. (1962) and Kagan and Breznock (1979) performed direct lymphangiography in the dog, using a radiopaque medium injected into the metatarsal and intestinal lymph vessels, respectively. Mulshine et al. (1987) developed an immunolymphoscintigraphic technique to study pulmonary and mediastinal lymph nodes as a new approach to lung cancer images. Clinical applications of lymphography include identification of lymph node lesions, evaluation of lymph vessel blockage, and visualization of the progress of therapy on lymphatic lesions (Fischer, 1959; Fischer & Zimmerman, 1959; Skelley et al., 1964; Suter, 1969). Research on the anatomy and physiology of the lymphatic system has resulted in a considerable body of knowledge, although many questions remain unanswered. Drinker (1942) commented on the observations of Gaspar Asellius (1581-1626) and of Pecquet (in 1651) on the lymph vessels and nodes in the dog. Asellius described and illustrated the mesenteric lacteals and mesenteric lymph node (“pancreas of Aselli”); Pecquet described the receptaculum chyli (“cistern of Pecquet”) and the thoracic duct, including its termination. Rusznyak et al. (1967) credit Rudbeck (1652) and Bartholinus (1653) for having recognized the lymphatic system as an entity. The English edition of the monograph on lymph vessels and lymph circulation by Rusznyak, Földi, and Szabo (1967), revised and enlarged from the Hungarian, German, and Russian editions, contains an extensive bibliography (more than 1700 citations). Frequent reference is also made therein to the morphologic features and experimental physiologic characteristics of the lymphatic system in the dog. Chretien et al. (1967) described the distribution of lymph nodes in the dog and discussed the effects of surgical excision of the thymus, spleen, and all gross lymph nodes on the survival of skin allografts. They injected Pontamine Blue intravenously 3 or more days prior to dissection and found that all lymphoid tissue was stained a deep blue. Excision of lymphoid tissue did not significantly alter the allograft response. A classic topographic description of the lymph nodes and vessels in the dog was written by Hermann Baum in 1918. Most of the illustrations used in this chapter have been reproduced from that work, Das Lymphgefässystem des Hundes, through the kind permission of Springer-Verlag, Berlin. The homologic characteristics of the lymphatic system of the dog and other domestic animals is discussed by, among others, Wilkens and Munster (1972) and Spira (1962), and Shdanow (1962) attempts to answer some of the contentious questions of his time on the functional morphology of the lymphatic system. The terminology of the present text conforms to the Nomina Anatomica Veterinaria (NAV) (2005). The innervation of lymph nodes and lymph vessels is well established. (For a review see Vajda [1966].) In addition to the findings of earlier works, Vajda (1966) established that the adventitia of lymph vessels is supplied by large-caliber, myelinated axons, that the muscular coat is supplied by a circular plexus of unmyelinated axons, and that lymph capillaries lack a nerve supply. However, Todd and Bernard (1974) could demonstrate only a loose network of sympathetic axons in the adventitia of the thoracic duct. In a study on the effect of vasoactive agents on hemorrhage, Dobbins et al. (1986) and Dabney et al. (1988) found that lymphatic pressure was significantly increased following hemorrhage, carotid occlusion, or the injection of vasoactive agents. This indicated that prenodal lymph vessels actively constrict in response to the neural or hormonal consequences of hemorrhage on lymph vessels or to the introduction of vasoactive substances, and that lymphatic function is subject to neural or hormonal regulation. Volik (1962) studied the inguinal lymph nodes and found that they were innervated by sympathetic and somatic axons. The central nervous system can modulate the immune response through the sympathetic and sensory innervation of lymphoid organs. Results of a study by Popper et al. (1988) indicate that sympathetic and sensory innervation of lymph nodes is involved with the regulation of their blood and lymph flow, and that the neuropeptide receptor binding sites in lymph node germinal centers may be expressed by lymphocytes on activation by antigens. The effect of hormonal control over lymph nodes was also demonstrated by Korovina (1985), who states that extirpation of the pancreas resulted in progressive involution of the immunocompetent tissue in the inguinal lymph nodes. Lymphoid tissue is present in all classes of vertebrates but reaches its highest development in mammals. Bryant (1975) used lymph node structure as an ontogenic explanation of divergence in Eutheria, Metatheria, and Prototheria. Under normal physiologic conditions lymphocyte accumulations occur in the lamina propria of mucous membranes of the digestive, respiratory, and urogenital tracts. Spherical lymphoid colonies are known as lymph nodules (nodulus lymphaticus) and are seen only in specific pathogen-free and newborn animals. On exposure to foreign substances, the primary nodules (nodulus primarius) change into secondary nodules (nodulus secondarius) characterized by reaction centers. Some secondary nodules lying in the lamina propria of the mucosa and extending into the submucosa are known as solitary nodules (nodulus lymphaticus solitarius). They occur in the digestive, respiratory, and urogenital tracts as well as in the conjunctiva and in the mucous membranes of all natural body orifices. Secondary nodules in the intestine also occur as large, connected accumulations and are then known as aggregated lymph nodules (noduli lymphatici aggregati) or Peyer patches. In the spleen, thymus, lymph nodes, hemal nodes, and tonsils the lymph nodules develop into circumscribed organs. Lymphocytes are produced mainly by hematopoietic tissue of bone marrow. Yoffey and Courtice (1956) estimate that the total amount of lymphoid tissue in the mammalian body is approximately 1% of the body mass. The proportional distribution and form of the lymphoid tissue in the various species of mammals vary greatly. The dog has but one or two large nodes at each nodal station. Lymph nodes (lymphonodi) are always located in the course of lymph vessels. The vessels that enter the node are known as afferent lymph vessels (vasa lymphatica afferentia). They break up into many minute vessels before perforating the capsule of the lymph node. After passing through subcapsular, cortical, and medullary lymph spaces or sinuses within the lymph node, they unite and leave the lymph node at the hilus as one or more efferent lymph vessels (vasa lymphatica efferentia). Probably all lymph vessels pass through at least one lymph node (Yoffey & Courtice, 1956). Some pass through several lymph nodes. The lymph vessels therefore form portal systems comparable to the venous portal system of the mammalian liver and the arterial portal system of the kidney in lower vertebrates. Certain lymphoid organs have only efferent lymph vessels. Examples of these are the tonsillar masses of the pharynx, and the solitary and aggregated lymph nodules in the mucous membrane of the digestive system. The spleen, thymus, and bone marrow are interposed not in the lymphatic system but rather in the blood vascular system. Lymphoid tissue, wherever found, probably reaches its greatest development at sexual maturity. Endocrine and sex differences affect lymphoid tissue. Although Korovina (1985) has shown that the inguinal lymph nodes undergo involution after extirpation of the pancreas, investigators are not in agreement about the effects of endocrine and sex differences on lymphoid tissue (Yoffey & Courtice, 1956). The thoracic duct (ductus thoracicus) (Figs. 13-2 through 13-4, and Figs. 13-9A through 13-13) is the chief channel for return of the lymph of the body. Lymph from the right thoracic limb and the right side of the neck and head is returned via the right lymphatic duct (ductus lymphaticus dexter). Normally the lymph vessel system joins the venous system at the “venous angle,” that is, the point of confluence of the external and internal jugular veins or the junction of the jugular and subclavian veins. The thoracic duct begins in the sublumbar region, or between the crura of the diaphragm as a cranial continuation of the cisterna chyli (see Figs. 13-2, 13-17, and 13-23). This cistern is a dilated, bipartite portion of the lymph channel that lies retroperitoneally in association with the cranial abdominal aorta. The dorsal part is saccular and lies dorsal to the aorta from the level of the origin of the celiac artery to approximately the level of the left renal hilus. Cranially it continues through the aortic hiatus of the diaphragm as the thoracic duct. The ventral part of the cistern lies on the ventral surface of the aorta, concealed in part by the caudal vena cava, and extends from the level of origin of the cranial mesenteric artery to just caudal to the caudal pole of the left kidney. The ventral part of the cistern is frequently plexiform and commonly receives paired visceral lymph trunks (truncus visceralis) from the abdominal viscera, and lumbar lymph trunks (trunci lumbales) that are the cranial continuations of the lymph vessels from the pelvis and pelvic limbs via efferent lymph vessels from the iliac lymph nodes. The ventral part of the cistern has a variable number of connections around the aorta to the dorsal part of the cistern (Lindsay, 1974). The afferent lymph vessels that drain into the cistern are covered by a fold of endothelium, and lack valves where they enter the cistern (Marais & Fossum, 1988). Magnetic resonance imaging in 30 dogs found that the cisterna chyli always wrapped around the aorta, but varied in size and shape (Johnson & Seiler, 2006). The exact origin of the thoracic duct is somewhat arbitrarily assigned, because the morphologic characteristics of the cisterna chyli are so erratic. The thoracic duct is considered to begin mostly as a single duct between the crura of the diaphragm, where the cistern attains its minimum width (de Freitas et al., 1981). Johnson and Seiler (2006) showed that the thoracic duct formed from multiple small branches around the aorta cranial to the diaphragmatic crura, which united to become one tubular structure. The initial part of the thoracic duct is plexiform and variable in form, whereas the distal part is more constant. Detailed descriptions of the variations in morphologic characteristics and topography of the thoracic duct are given by de Freitas et al. (1981), Kagan and Breznock (1979), and Threefoot (1968). Huber (1909) compared the thoracic ducts of the horse, ox, dog, and pig and illustrated 20 variations in the origin, course, or termination of the thoracic duct of the dog (see Fig. 13-2). The thoracic duct runs cranially from a point opposite the first lumbar vertebra on the right dorsal border of the thoracic aorta and the ventral border of the azygos vein to the sixth thoracic vertebrae. Here it inclines to the left, running between the azygos vein and the aorta, and then obliquely crosses the ventral surface of the fifth thoracic vertebra to enter the cranial mediastinum. It continues cranioventrally in the cranial mediastinum, and in approximately one half of specimens it terminates singly at the junction of the left external jugular vein with the left subclavian vein (Baum, 1918). In many specimens the cranial portion of the thoracic duct bifurcates or trifurcates. These branches are usually connected by cross branches so that a coarse, widespread plexus is formed. The termination of the end parts of the thoracic duct varies as to both the veins they empty into and the places on the vessels at which they empty. When the thoracic duct is single, it usually presents a terminal dilatation immediately before it becomes constricted to perforate the venous wall. Similar ampulla-like dilatations may be present on each of the branches when the thoracic duct terminates in multiple vessels. A monocuspid valve is present at the thoracic duct opening into the venous system. Immediately distally, the lymphaticovenous junction is closed by an extensive, convoluted, bicuspid valve (Marais & Fossum, 1988). Freeman (1942), who examined the termination of the thoracic duct in 25 dogs, found that it had no branches in three and was divided in five, and that it sent branches to the right side in eight, to the azygos vein in five, and to both the right side and the azygos vein in four. Suter and Greene (1971) describe a case of chylothorax in a dog with an abnormal termination of the thoracic duct. There is a connection between the thoracic duct and the right lymphatic duct in 50% of the dogs (Martin et al., 1983; Pieper et al., 1986; Vreim et al., 1977). The right and left tracheal trunks (truncus trachealis), 2 to 4 mm wide, arise from the caudal pole of the ipsilateral medial retropharyngeal lymph node or its efferent lymph ducts. The tracheal trunk is usually single as it leaves the caudal pole of the lymph node, but it may be double or in the form of a plexus. As it runs caudally in the neck, it lies in or adjacent to the medial wall of the carotid sheath. No additional nodes are found along the course of the tracheal trunk (Yoffey & Drinker, 1938). The left tracheal trunk usually terminates in the thoracic duct. The right tracheal trunk usually terminates in the right lymphatic duct. The right lymphatic duct (ductus lymphaticus dexter) is located in the right caudal part of the neck. It receives efferent lymph vessels from the right superficial cervical, axillary and thoracic lymph nodes. The right tracheal trunk drains into the right lymphatic duct 20-30 mm from the first rib, doubling the size of it (Baum, 1918). After a course of approximately 15 mm, the right lymphatic duct empties into the right subclavian vein or the angle formed by the merging of the right subclavian and right external jugular veins. Commonly, the right lymphatic duct bifurcates and then reunites, forming a circle before it terminates. Sometimes the forked condition persists, so that the right lymphatic duct enters the venous system, cranial to the first rib, by two channels. The right lymphatic duct drains into the thoracic duct in 50% of dogs (Martin et al. 1983; Pieper et al., 1986; Vreim et al., 1977). Although these ducts are among the larger lymph ducts of the body, the smooth muscle cell component is sparse. A network of sympathetic axons coming from the plexus on blood vessels outside the carotid sheath are present on the surface of the cervical lymph ducts in dogs (Todd & Bernard, 1973). The paucity of contractile cells would however indicate that intrinsic contraction is not a prime mechanism in the propulsion of lymph (Todd & Bernard, 1974). The parotid lymph center (lymphocentrum parotideum) consists of the parotid lymph nodes (lymphonodi parotidei superficiales) (Figs. 13-1, 13-5, and 13-6) at the rostral base of the ear. They are beanshaped nodes located deep to the rostrodorsal border of the parotid salivary gland on the caudal parts of the zygomatic arch and adjacent masseter muscle. In a medium-sized dog they are approximately 10 mm long, 5 mm wide, and 3 mm thick. Occasionally, a second or even a third node may be present (Baum, 1918). They are palpable in this position when enlarged. l. Caudal belly of m. digastricus s″ Lateral retropharyngeal lymph node u. Medial retropharyngeal lymph node 1, 1′ Lymph vessels of the hard and soft palate 1′ Lymph vessels of the zygomatic gland that join 1′ 2. Lymph vessels of the soft palate 2′ Lymph vessel of fold of tonsillar sinus 3. Lymph vessels of the tonsil 4, 4′, 4″ Lymph vessels of the soft palate, the tonsil, the base of the tongue, and the mucous membrane of the nasopharynx, which pass between the mucous membrane and muscles 5. Lymph vessels of the tongue that go through the m. hyoglossus 6, 6′, 6″ Lymph vessels of the tip of the tongue 7, 7′, 8. Lymph vessels of the body of the tongue a. M. mylohyoideus (reflected) f. Caudal belly of m. diagastricus (cut) l. M. sternocephalicus, pars mastoideus and m. cleidocephalicus, pars mastoideus (each partially removed) p, q. Parotid and mandibular salivary glands (each partially removed) r, r′ Monostomatic sublingual salivary glands t. Medial retropharyngeal lymph node u. Lateral retropharyngeal lymph node The mandibular lymph nodes (lymphonodi mandibulares) (see Figs. 13-1, 13-5, and 13-6) form a group of two or three nodes or, rarely, as many as five that lie ventral to the angle of the mandible. A flattened, three-sided node, with borders approximately 10 mm long, lying dorsal to the linguofacial vein and a long, ovoid node lying ventral to the linguofacial vein constitute what is probably the most common arrangement. The node lying ventral to the linguofacial vein usually is more than 20 mm long and approximately 10 mm wide. It is flattened transversely. In more than one third of the specimens examined by Baum (1918), two or more nodes replaced this single node on one or both sides. In only 16 of 36 specimens was the grouping of the mandibular nodes on the two sides similar. The dorsal node may be double, but this condition is rare. Drainage Area. The afferent lymph vessels to the mandibular lymph nodes come from all parts of the head not drained by the afferent lymph vessels of the parotid lymph node. There is overlapping in the areas of drainage, so that the eyelids and their glands and the skin of the dorsum of the cranium and the temporomandibular joint drain into both nodal centers. Maher (1986) found that a mucosal and submucosal lymphatic network drained the palate. The afferent lymph vessels exit the palatal area and subsequently join lymphatic networks intrinsic to the tongue and pharynx. Maher (1985) and Kostiuk (1986) have shown that superficial and deep lymph capillary networks drained the tongue. Furthermore, Ossoff et al. (1980) and Ossoff and Sisson (1981) have shown that a superficial and a deep lymph capillary network drained the floor of the oral cavity. The superficial lymph vessels randomly crossed the midline and drained into either the ipsilateral or the contralateral mandibular lymph nodes. However, the deep lymph vessels drained through the periosteum of the mandible and then into the mandibular lymph nodes, or along the medial surface of the mandible and then into the medial retropharyngeal lymph nodes. The efferent lymph vessels of the mandibular lymph nodes go primarily to the ipsilateral medial retropharyngeal lymph node. The lymph nodes composing the group are connected with each other as well as with the contralateral medial retropharyngeal lymph node. The 8 to 10 efferent lymph vessels anastomose with each other and form a plexus as they pass over the pharynx. Before reaching the medial retropharyngeal node, they unite to form three to five small trunks that enter the ventrolateral surface of the medial retropharyngeal lymph node. According to Battezzati and Donini (1973), Bronzini in 1935 injected India ink into the cerebellomedullary cistern of a dog and observed the ink in the nasal mucosa, pharynx, and mandibular lymph nodes. This communication between the subarachnoid spaces and the lymph vessels of the nasal mucosa and pharynx may be of considerable importance in disease processes. The buccal lymph nodes (lymphonodi buccales) are situated dorsal, ventral, or rostral to the angle of confluence of the facial and superior labial veins, dorsal to the buccinator muscle. The flattened nodes are approximately 10 mm long and 5 mm wide and can be bilateral, unilateral, or absent. Shelton and Forsythe (1979) examined 250 dogs of various breeds and found unilateral lymph nodes in 11 dogs, and bilateral lymph nodes in 11 dogs. Rumph et al. (1980) examined 171 Greyhounds and found lymph nodes to be present in 15 of them, and Casteleyn et al. (2008) found 6 bilateral and 7 unilateral buccal lymph nodes in 150 dogs that they examined. The medial retropharyngeal lymph node (lymphonodi retropharyngei mediales) (Figs. 13-5 through 13-8) is the largest node found in the head and neck. It is an elongated, transversely compressed node, with a more pointed caudal end, and is approximately 50 mm long and nearly 20 mm wide. Burns et al. (2008) found that the node increased in size with increased body weight, but decreased in size with increased age. The medial retropharyngeal lymph node lies ventral to the wing of the atlas in the triangle bounded by the m. digastricus cranially, the m. longus colli dorsally, and the pharynx and larynx ventromedially. The mastoid parts of both the m. cleidocephalicus and the m. sternocephalicus largely cover the lymph node laterally, although its cranioventral part is related to the mandibular salivary gland. Coursing along its medial surface is the hypoglossal nerve and the terminal portion of the carotid sheath with its common carotid artery, vagus and sympathetic nerves, and the internal jugular vein. In 10 of 47 specimens, Baum (1918) found two medial retropharyngeal lymph nodes present on one or both sides. The afferent lymph vessels of the medial retropharyngeal lymph node come from all the deep structures of the head that have lymph vessels. Thus the tongue; the walls of the oral, nasal, and pharyngeal passages; the salivary glands; and the deep parts of the external ear drain into this lymph node. It also receives afferent lymph vessels from the larynx, esophagus, and the noncutaneous, nonmucous structures of the neck that have lymph vessels. The efferent lymph vessels from the parotid, mandibular and lateral retropharyngeal lymph nodes drain into the medial retropharyngeal lymph node. Belz and Heath (1995) showed that efferent lymph vessels from the mandibular lymph nodes convey lymph to defined areas in the medial retropharyngeal lymph nodes. Yoffey and Drinker (1938) found four or five lymph vessels, lying between the hamulus of the pterygoid bone and the pharyngeal opening of the auditory tube, that came from the floor and sidewalls of the nasal cavity. The deep lymph vessels of the nasal cavity drain into the retropharyngeal lymph nodes and pass via the tracheal trunks to the region of the thoracic inlet veins. Some of the tracheal lymph vessels of the neck communicate with bronchial lymph vessels and reach the bronchial lymph nodes. Thus it is possible for lymph from the nasal cavity to reach the lung area. a, a′ Medial retropharyngeal lymph nodes b. Cranial deep cervical lymph node c, c′ Caudal deep cervical lymph nodes d, d′, d″ Superficial cervical lymph nodes e′ Accessory axillary lymph node g. Efferent vessel of the superficial cervical lymph nodes i. Thoracic duct with terminal branches k-k′″. Lymph vessels of the larynx l. Lymph vessel that runs to a cranial mediastinal lymph node m to m3. Mandibular lymph nodes n. Efferent vessels of the mandibular lymph nodes that go to the medial retropharyngeal lymph node of the opposite side Lateral retropharyngeal lymph nodes (lymphonodi retropharyngei laterales) are present in approximately 30% of dogs (Baum, 1918) (Figs. 13-5 and 13-6). This lymph node is less than 10 mm in diameter and lies at the dorsal border of the horizontal part of the cartilaginous external acoustic meatus. It is completely or partially covered by the caudal part of the parotid salivary gland. The superficial cervical lymph center (lymphocentrum cervicale superficiale) consists of the superficial cervical lymph nodes (lymphonodi cervicales superficiales) (Figs. 13-1, 13-7, and 13-8). They usually consist of two nodes, one lying dorsal to the other in the adipose tissue on the lateral surface of the serratus ventralis and scalenus muscles, cranial to the supraspinatus muscle. They are covered superficially by the thin cleidocephalicus, omotransversarius, and, at their dorsal end, the trapezius muscles. The superficial cervical artery and vein lie medial to the caudal parts of the lymph nodes as they course cranial to the shoulder in the groove between the shoulder and the neck. The more ventral superficial cervical lymph node may encroach on the trachea on the right side, and the esophagus and trachea, on the left side. Occasionally, a single node is present on each side, but more commonly three or more nodes replace the usual two. Most of the nodes are oval and somewhat flattened. They are collectively approximately 30 mm long and less than 10 mm thick. Drainage Area. The afferent lymph vessels come mainly from the skin of the caudal part of the head, including the pharyngeal region, a part of the pinna, the lateral surface of the neck, and the whole thoracic limb, except a variable region on the medial side of the brachium and antebrachium, the shoulder, and the cranial part of the thoracic wall. Yang (1986) found that one or occasionally both nodes showed green coloration after tattooing the ear. Mahorner et al. (1927), by making injections into the thyroid gland of dogs, revealed efferent connections with the superficial cervical lymph nodes, the tracheal trunks, and the veins at the base of the neck. The efferent lymph ducts connect members of the group when more than one node is present, and as one to three trunks they descend over the serratus ventralis and scalenus muscles to merge with the tracheal trunk on the right side, contributing to the right lymphatic duct. On the left side the superficial cervical lymph nodes empty into the thoracic duct. On either or each side they may empty into the external jugular vein directly. Efferent lymph vessels from the first thoracic mammary gland usually entered the axillary lymph node, but may also enter the superficial cervical lymph nodes (Patsikis & Dessiris, 1992).
The Lymphatic System
General Considerations
Ontogenesis of the Lymphatic System
Lymph Drainage
Locomotor System
Central Nervous System
Serous Membranes
Digestive Tract
Respiratory System
Urinary System
Circulatory System
Lymph Vessels
Innervation of Lymph Vessels and Lymph Nodes
Lymphoid Tissue
Lymph Nodes
Regional Anatomy of the Lymphatic System
Large Lymph Vessels
Lymph Nodes and Vessels of the Head and Neck
Parotid Lymph Center
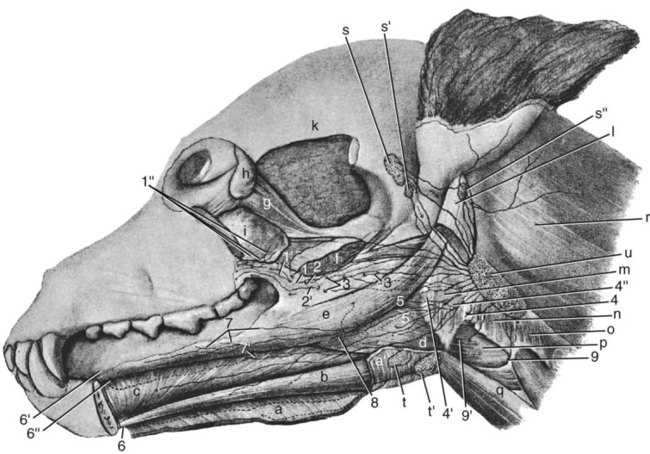
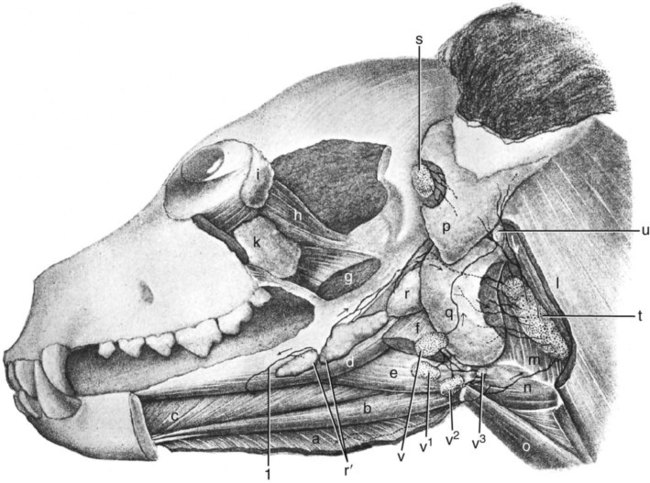
Mandibular Lymph Center
Retropharyngeal Lymph Center
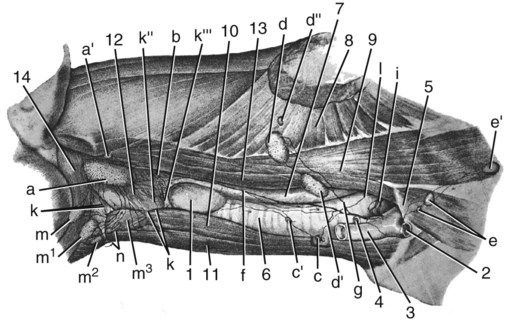
Superficial Cervical Lymph Center
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Lymphatic System
Only gold members can continue reading. Log In or Register to continue