Chapter 11
The Lymph Nodes
Architecture
When interpreting a cytologic specimen of the lymph node, it is useful to keep in mind the histologic structure and different cell types that are found in this tissue. The node is composed of a capsule, cortex, medulla, and sinuses (subcapsular, cortical, and medullary).1 The cortex, or the more peripheral area of the node, is divided into follicular and diffuse (parafollicular cortex or paracortex) regions, and the medulla, or the more central area, is divided into the medullary cords and sinuses (Figure 11-1).
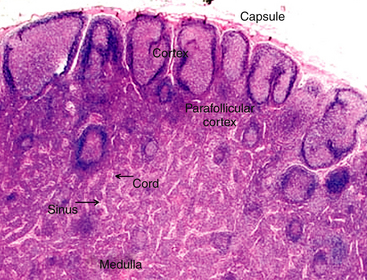
Figure 11-1 The lymph node has two basic parts: the cortex and the medulla.
The cortex has both follicular and diffuse or parafollicular regions. The parafollicular region gradually transforms into medullary cords of B-lymphocytes and plasma cells, which are surround by sinuses containing macrophages attached to reticular fibers. Different populations of lymphocytes in these areas and other cells are found in nodal aspirates. (H&E stain.)
Lymph nodes are strategically located at sites throughout the body and are involved in a variety of local and systemic disease processes. Antigen reaches the node via the afferent lymphatics. The lymph percolates through the sinus and sinusoidal walls into the parenchyma (Figure 11-2), where foreign substances (antigens) are taken up and processed by specialized cells of the MPS. The sinuses (subcapsular, cortical, and medullary) form a network of branching channels that converge at the hilus of the node to exit by the efferent lymphatics. The primary functions of the lymph nodes include filtering particles and microorganisms, exposing antigens to circulating lymphocytes, and activating B- and T-lymphocytes. The superficial, subcutaneous location of some lymph nodes (mandibular, superficial cervical, inguinal, and popliteal) allows easy detection of enlargement and access for fine-needle aspiration (FNA) cytology. It is appropriate to aspirate any node that is enlarged, and in the case of lymph nodes draining areas affected by neoplasia, even in the absence of enlargement, aspiration may be justified.2
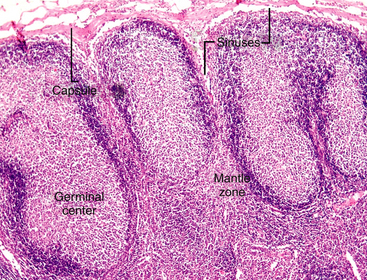
Figure 11-2 Lymph enters the node via the afferent lymphatics, percolating through subcapsular sinuses and sinusoids, where foreign substances (antigens) are taken up and processed. The secondary follicles in this node have a peripheral rim or mantle zone and a pale-staining, central germinal center. The differentiation of B-lymphocytes in response to antigen occurs in the germinal center of the follicular cortex. (H&E stain.)
General Considerations
Lymph node aspiration cytology has become a popular procedure in human medicine in recent years, because of its great convenience.3 Similarly, this high-yield diagnostic technique is frequently used in veterinary medicine.4–7 A few points need to be considered when obtaining nodal samples for cytologic evaluation.
A normal lymph node is small and often difficult to aspirate. It is not uncommon for the cytology of a normal node to contain mostly perinodal adipose tissue and only a few or no lymphocytes. If multiple nodes are enlarged, then sampling from several nodes is recommended. Because the submandibular nodes drain the oral cavity, they often become enlarged, reactive, inflamed, or all of these may occur. A confusing mixture of malignant, reactive, and inflammatory cells may limit the accuracy of cytologic diagnosis of lymphoma based on FNA of these nodes. Thus, sampling of submandibular nodes should be avoided in cases where generalized lymph node enlargement exists. The prescapular and popliteal nodes are often a better choice. The mandibular salivary gland is quite frequently mistaken for a node and aspirated. However, the presence of large, foamy epithelial cells, either individually or in clusters, and mucus in the background allows the salivary tissue to be easily identified.
Consideration also should be given to the size of the lymph node when deciding which node to aspirate —very large nodes may have areas of hemorrhage or necrosis. If the node must be sampled, the needle should be directed tangentially, avoiding the more central portions.8 Finally, when obtaining a sample for cytologic evaluation, it is important to remember that the lymph node is a heterogeneous tissue, and multiple areas within the node should be sampled to be certain that what has been obtained is representative. While keeping the needle in the node, the needle should be repeatedly advanced and withdrawn in multiple directions until a small amount of aspirate appears in the hub of the needle. This procedure may be done using a syringe to apply gentle suction or with only the needle. If the former technique is used, the suction should be released before removing the needle from the node. Overly vigorous aspiration of the lymph node produces significant hemodilution and cells may rupture, limiting the interpretation of the sample. A large volume of aspirate is not required; the material within the hub of the needle is sufficient for making cytologic preparations. Because the lymphocytes are fragile, care must be taken to apply only minimal pressure when making slide preparations to prevent excessive rupturing of cells. The slides are air-dried (not heat-fixed) and stained for evaluation.
Fine-Needle Aspiration
FNA is a relatively safe and painless procedure, allowing for rapid and inexpensive sampling of peripheral lymph nodes. It does not require hospital admission or anesthesia of the pet. The role of this procedure is summarized in Box 11-1.
Cytologic Findings
Normal Lymph Node
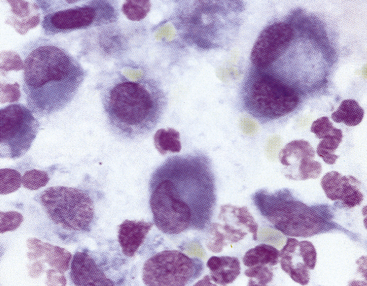
In the absence of architectural features that can be appreciated in a histologic section of a lymph node, the interpretation of cytology relies on proportions of different cell types and an understanding of what proportions are normal versus abnormal for these cell types. Small, well-differentiated lymphocytes compose greater than 75% to 85% of the total nucleated cell population (Figure 11-3 to Figure 11-7).4–7 They have round nuclei that are about 1 to less than 1.5 times the size of a mature red blood cell (RBC), with an overall cell size that is smaller than that of a neutrophil. Their chromatin is densely clumped, and nucleoli are not visible. The nucleus-to-cytoplasm (N:C) ratio is high, with a narrow rim of basophilic cytoplasm. In addition to small lymphocytes, a normal node should have low numbers (<10% to 15%) of lymphocytes that are intermediate to large (often called lymphoblasts) in size; their nuclei are 1.5 to 3 times the size of an RBC, with an overall size ranging from about that of a neutrophil or larger to up to 4 times the size of an RBC (see Figure 11-5). Their chromatin is less clumped, and nucleoli may be visible and even multiple, prominent, or both. The cytoplasm is pale blue and more abundant than in small lymphocytes.
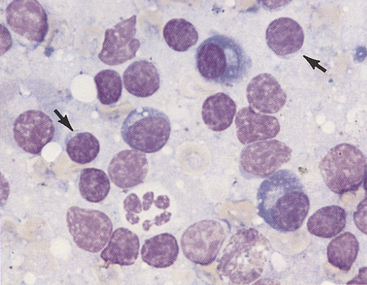
Figure 11-3 Aspirate from a hyperplastic lymph node.
Small lymphocytes (arrows) and plasma cells characterize the reaction. Note that the small lymphocytes are smaller than the neutrophils and their nuclei are about the size of a red blood cell. Many free nuclei, identified by pink, homogeneous chromatin and an absence of cytoplasm, are evident. (Wright stain.)
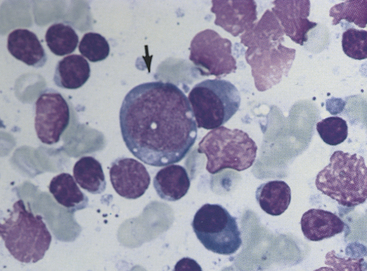
Figure 11-4 Small lymphocytes, plasma cells, and a large transformed lymphocyte (arrow) characterize this aspirate from a hyperplastic lymph node. Irregular, pink nuclei from lysed cells are seen. (Wright stain.)
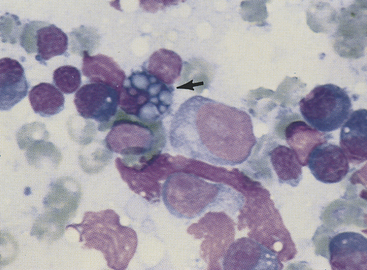
Figure 11-5 Plasma cells, small lymphocytes, and two large lymphocytes (lymphoblasts) characterize this aspirate from a hyperplastic lymph node. The plasma cell with the vacuolated cytoplasm is a Mott cell containing Russell bodies (arrow). The pink, amorphous structures are free nuclear material. (Wright stain.)
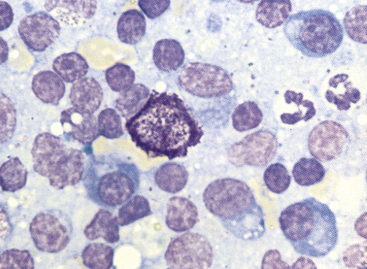
Figure 11-6 Small lymphocytes, plasma cells, neutrophils, and a single mast cell are present in this aspirate from a hyperplastic lymph node. (Wright stain.)
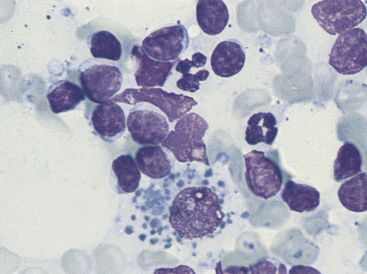
Figure 11-7 Seen among small and medium-sized lymphocytes is a large macrophage containing phagocytized debris. (Wright stain.)
Plasma cells have small, round, eccentric nuclei with condensed chromatin (see Figures 11-3 to 11-6). Their abundant cytoplasm is deep blue and has a prominent, clear Golgi zone. Immature plasma cells (transformed B-lymphocytes) are larger and have less aggregated chromatin and a higher N:C ratio (see Figure 11-4). Their very blue cytoplasm may contain discrete vacuoles. Plasma cells in various stages of development are seen in small numbers in normal lymph nodes, but typically represent less than 3% of the total nucleated cell population.
Macrophages, characterized by abundant cytoplasm often containing vacuoles and granular debris, also are found in small numbers (see Figure 11-7). Macrophages from areas of intense lymphopoiesis and cellular turnover may contain prominent basophilic nuclear debris (tingible bodies) (Figure 11-8). Occasionally, small numbers of neutrophils, eosinophils, and mast cells are observed in a normal node (see Figure 11-6). Each of these cell types should represent less than 1% of the cell populations in a normal node. It is important to consider the amount of blood contamination when making this assessment. Reticular cells and endothelial cells are common in lymph nodes, but these tissue-bound cells are rarely aspirated intact. They usually appear as large swollen nuclei, often devoid of cytoplasm (Figure 11-9).
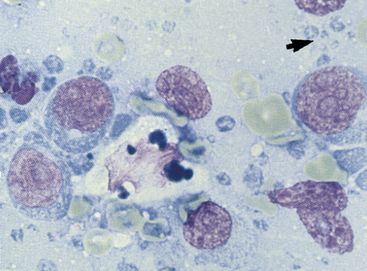
Figure 11-8 In this aspirate from a lymphomatous lymph node are large immature lymphocytes with prominent nucleoli, dispersed chromatin, and abundant blue cytoplasm. The large cell in the center with bluish cytoplasmic globules is a tingible-body macrophage. The small blue structures (arrow) are lymphoglandular bodies. (Wright stain.)
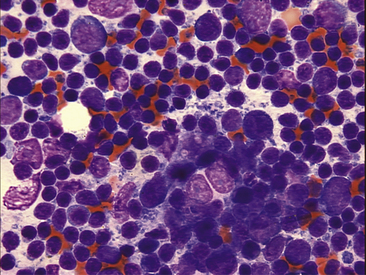
Figure 11-9 Several reticuloendothelial cells are seen in this lymph node aspiration cytology. Their nuclei are swollen, and they are devoid of cytoplasm. Small lymphocytes are the predominant cell type in this mildly hyperplastic node.
Because of the pressures of the aspiration technique, lymphocytes, which are very fragile, may rupture and release their nuclei. Free nuclei are swollen and uniformly pink in contrast to the blue blocky or granular pattern of intact lymphocyte nuclei (see Figures 11-3 to Figure 11-5). Blue nucleoli are often exposed in the nuclear chromatin of ruptured cells. These free nuclei carry no diagnostic significance and should not be confused with large immature lymphocytes. Lymphoglandular bodies are cytoplasmic fragments and are highly characteristic of lymphoid tissue (see Figure 11-8; Figure 11-10). They are round, homogeneous, basophilic structures that are similar in size to platelets.
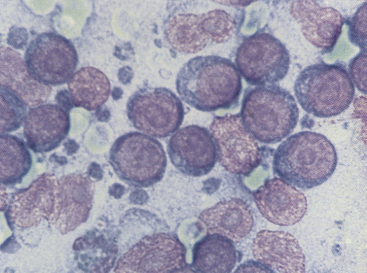
Figure 11-10 Immature, neoplastic lymphocytes characterize this smear from a dog with malignant lymphoma. Note that the cells are much larger than red blood cells. The pink structures lacking cytoplasm and containing prominent nucleoli are nuclei from lysed cells. Small blue lymphoglandular bodies are numerous. (Wright stain.)
Lymphadenopathy
One of the most common indications for performing aspiration cytology is enlargement of one or more lymph nodes. Three general processes cause lymph node enlargement: (1) inflammation (lymphadenitis—suppurative, pyogranulomatous, granulomatous, and eosinophilic), (2) immune stimulation (hyperplasia or reactive lymphadenopathy), and (3) neoplasia (lymphoma or metastatic). The cytologic evaluation of a sample obtained by FNA or touch imprint from the excised node usually allows differentiation of these processes. However, these processes are not mutually exclusive and may occur simultaneously.
Lymphadenitis
Lymphadenitis or inflammation of the node may be a primary (node itself is inflamed or necrotic) or secondary (node is draining an area of inflammation or necrosis) finding. This process is characterized cytologically by accumulation of inflammatory cells. Neutrophils, eosinophils, and macrophages occur singly or in combination. Inflammation is probably present when the population is greater than 5% neutrophils or greater than 3% eosinophils, provided no significant blood contamination has occurred. Macrophage numbers may increase in inflammation but also in hyperplasia and sometimes in neoplasia. Macrophages also may appear as epithelioid cells and multinucleated giant cells in granulomatous inflammation. Epithelioid macrophages are characterized by blue cytoplasm with minimal vacuolization and contain very little phagocytic debris (Figure 11-11). Organisms may, however, be present within the cytoplasm. These cells may occur in aggregates. Inflammatory cells may represent only a small portion of the total cell population, which otherwise suggests lymphoid hyperplasia, or they may completely replace the normal cell population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree