Chapter 17
The Lung and Intrathoracic Structures
Sample Collection
Equipment and Technique
Intrathoracic FNA is performed using 20- to 25-g (most commonly 22-g) needles of variable length, depending on the size and depth of a lesion. Superficial lesions may usually be sampled using a 1.5-inch needle. A 2.5- to 3.5-inch (spinal) needle may be needed to reach lesions in deeper locations. A 5- to 12-mL (milliliter) syringe is attached to the needle either directly or by means of an extension tube. Additional equipment needs include glass slides with or without culture media.1
The procedure is performed after a lesion has been identified, most often by means of thoracic radiographs. Although diffuse intrathoracic lesions such as diffuse, infiltrative pulmonary disease may be amenable to blind sampling, image guidance is invaluable in obtaining targeted samples from focal or multifocal lesions associated with mediastinal structures and the pleura or pleural space, thoracic wall, diaphragm and lung.2–4 Imaging modalities most commonly used to obtain intrathoracic tissue samples in veterinary patients include fluoroscopy, ultrasonography, and computed tomography (CT).5–12 Although magnetic resonance imaging (MRI) is increasingly utilized in veterinary medicine, MRI-guided tissue sampling is, at this point, not commonly performed because of the need for expensive specialized nonferromagnetic biopsy equipment. Depending on the individual patient and the method used for tissue sampling, the procedure may be performed under sedation or may require general anesthesia. Intubation and breath-hold techniques are recommended when sampling small or deeply located lesions to minimize adverse effects from sliding of aerated lung against the needle during respiration. The thoracic wall at the sampling site is clipped and thoroughly cleaned of hair and debris (e.g., by scrubbing the skin with a soap-based disinfectant and alcohol). Samples can be obtained using aspiration or nonaspiration (“pincushion”) technique.13,14 Following the procedure, the needle is withdrawn, the sample is gently expelled onto a glass slide, and smears are prepared in a routine fashion.1
Special Considerations for Image-Guided Fine-Needle Aspiration
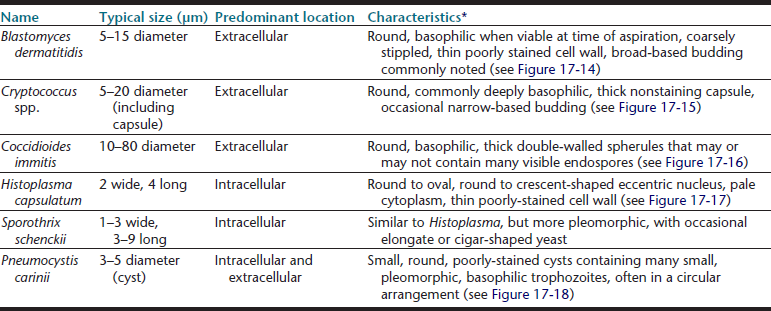
1. Ultrasonography is an excellent, inexpensive, and noninvasive modality to obtain guided fine-needle aspirates, which allows monitoring of needle placement in real time.6,7,9,11 The major disadvantage of this method is that ultrasound is unable to penetrate aerated lung tissue or free air in the pleural space. Ultrasound-guided sampling is, therefore, limited to superficial lesions in contact with the thoracic wall (Figure 17-1). Especially for small and deep-seated lesions, use of a needle guide is strongly recommended. Needle guides consist of probe attachments and needle channels, which dictate the course of the needle in a predetermined direction, thus minimizing risk of injury to surrounding structures and assuring needle placement in the area of interest (Figure 17-2).
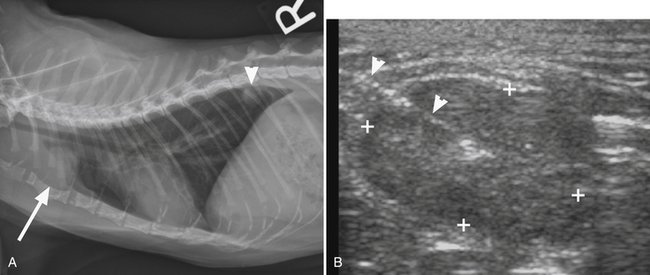
Figure 17-1 A, Lateral thoracic radiosgraph, cat, cranial mediastinal mass (arrow). Note also a pulmonary nodule in the caudal lung fields (arrowhead). B, Ultrasonographic image of same case, obtained during ultrasound-guided sampling of the mass. The needle is recognized as a strongly hyperechoic line entering the oval mass from the left top corner of the image (arrowheads). The cytology results were suggestive of a malignant fibrous histiocytoma.
Figure 17-2 Ultrasonographic image demonstrating use of a needle guide prior to ultrasound-guided aspiration of a pulmonary nodule.
The guide is attached to the probe and dictates course of the needle in a predetermined direction indicated by the dotted lines. Use of this technique minimizes risk of injury to surrounding structures and assures appropriate needle placement.
2. Fluoroscopy allows intermittent radiographic monitoring of needle placement as it is advanced toward a lesion.8 This technique has shown initial promising results, although potential radiation exposure to the clinician and necessity to rotate the patient or x-ray tube to achieve an orthogonal view to verify needle placement are disadvantages in comparison to CT.
3. CT is a cross-sectional imaging technique, which allows visualization of deep intrathoracic lesions and controlled placement even of long needles.10,12 Following a diagnostic scan of the thorax, a table (scan) position that is deemed most suitable for needle placement (usually at the largest extent of the lesion and avoiding vascular structures) is identified. After identification of the target, a laser light in the CT gantry is switched on to indicate the site of needle placement in the long axis of the patient. Additionally, marking the skin with ink, radiopaque markers (barium strips), or both prior to inserting the needle may be helpful to ensure appropriate needle placement. After the insertion site for the needle is identified, repeat CT images are obtained at the same table position to monitor advancement of the needle until the lesion is reached (Figure 17-3, A).
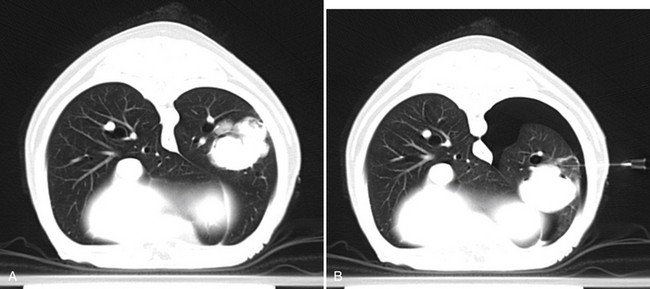
Figure 17-3 A, Transverse computed tomography image, dog, large left caudal lung lobe mass. A needle was advanced into the mass at this predetermined location and aspirates were obtained. B, Repeat image at the same level during a second attempt of fine needle aspiration reveals a moderate left-sided pneumothorax as indicated by a hypoattenuating (black) area surrounding the left lung. Note also change in position of the pulmonary mass secondary to atelectasis, which resulted in placement of the needle dorsal to the current position of the mass. Cytologic diagnosis of the mass was carcinoma. The pneumothorax did not result in clinical signs and resolved without treatment.
Complications
FNA of intrathoracic structures is generally considered a safe procedure. Potential complications include pneumothorax (see Figure 17-3, B) and hemorrhage, which are usually mild and self-limiting.1,8,12,15 More severe cases of pneumothorax may require placement of a thoracic drain, and animals should be monitored closely for dyspnea or other clinical signs following the procedure. One case of death from severe pneumothorax following fluoroscopy-guided aspiration of a thoracic lesion has been reported.8 Needle-tract implantation during FNA of thoracic lesions is extremely rare but has been reported in a dog with a pulmonary carcinoma.16
Diagnostic Yield
General diagnostic limitations of cytopathology in any tissue (e.g., lack of tissue architecture, varying cellularity of samples, limited ability to speciate certain organisms) apply to FNA cytology of the lung. Although a rate of nondiagnostic samples as high as 35% has been reported, FNA of thoracic lesions is considered a useful technique with a sensitivity ranging from 77% to 91% and a specificity as high as 100%.8,11,12,17,18 FNA cytopathology of the lung correlates reasonably well (82%-83%) with histopathology.3,18
Cytology of the Lung
FNA of lung tissue is most often performed when pulmonary lesions are detected using various imaging modalities (previously discussed). Specific indications include (1) presence of a focal pulmonary lesion; (2) presence of diffuse pulmonary parenchymal disease; (3) suspicion of primary pulmonary neoplasia; (4) suspicion of pulmonary metastatic disease; (5) need to obtain material for microbial culture; and (6) avoidance of more invasive procedures (e.g., biopsy, thoracotomy).2,19 For diffuse lesions, aspiration of the caudal right lung lobe is recommended.20 Ultrasound guidance (as previously discussed) may help minimize complications and maximize diagnostic yield, particularly when a focal lesion is present. Contraindications for FNA of lung tissue include bleeding disorders, bullous emphysema, fractious or otherwise uncontrollable patients (sedation may be needed to achieve adequate restraint), severe uncontrolled coughing, and pulmonary hypertension.21
Normal Lung
Samples from healthy pulmonary tissue are typically contaminated with a variable amount of blood and may contain small amounts of mucus, which appears as foci of extracellular, pink to lightly basophilic, amorphous or ribbonlike material (Figure 17-4). Nucleated cells are typically present in very low numbers and are predominantly well-differentiated, respiratory epithelial cells. Epithelial cells are arranged individually or in small clusters and may have cilia on their apical surface. They are cuboidal to columnar with lightly basophilic, finely granular cytoplasm and a single, round, eccentric (basal) nucleus with condensed or coarsely stippled chromatin (Figure 17-5). In addition to respiratory epithelium, low numbers of scattered (alveolar) macrophages are also commonly observed. Occasionally, very low numbers of goblet cells may be present. Goblet cells are columnar (or occasionally round) and frequently contain many pale, dark-pink or blue, discrete, cytoplasmic mucin granules (Figure 17-6). The nucleus of the goblet cell (when not obscured by its cytoplasmic granules) is small, round to oval, and eccentrically located. Occasional mucin granules may be present extracellularly and should not be confused with microorganisms or mast cell granules. The number of leukocytes present should not significantly exceed that which is expected with blood contamination.
Figure 17-4 Transtracheal wash, dog.
Low numbers of poorly preserved, mixed nucleated cells within a pink, granular background matrix are scattered around a moderate amount of amorphous to ribbonlike, ice-blue material consistent with mucus. (Wright stain. Original magnification 200×.)
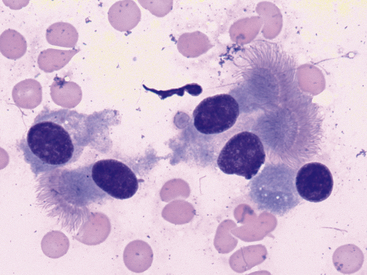
Figure 17-5 Lung FNA, dog.
Uniform, well-differentiated, columnar epithelial cells with an oval, eccentric (basal) nucleus, and apical cilia are the predominant cell type in samples from healthy pulmonary tissue. (Wright stain. Original magnification 1000×.)
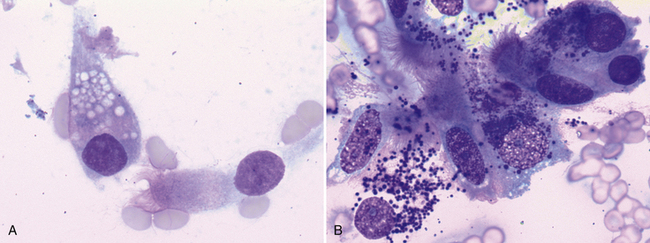
Figure 17-6 Goblet cells are a normal resident population in pulmonary tissue and are typically present in fewer numbers than ciliated columnar epitheliocytes. Goblet cells are columnar to round and contain many pale (A), dark-purple (B), blue, or magenta mucin-containing cytoplasmic granules. A, Nasal mass, FNA, dog. A single goblet cell with pale, cytoplasmic granules is next to a ciliated, columnar epithelial cell. (Wright stain. Original magnification 1000×). B, Lung mass, FNA, cat. Ciliated columnar epithelial cells are loosely aggregated with two intact goblet cells which contain many small, dark purple cytoplasmic granules. A third goblet cell is disrupted and several mucinous granules are scattered extracellularly. (Wright stain. Original magnification 1000×.)
Common contaminants of pulmonary samples include those observed for many other tissues—for example, ultrasound gel, cornstarch (glove powder), and skin contaminants (squamous cells, keratin bars, low numbers of bacteria not associated with inflammatory cells). However, samples collected via transthoracic lung FNA may also contain elements from the pleura (mesothelial cells), pleural space (substances from effusion, if present), or both (Figure 17-7). Additionally, well-differentiated hepatocytes (Figure 17-8), skeletal muscle (Figure 17-9), or both are sometimes observed. These findings are most consistent with inadvertent aspiration of the liver, diaphragm, or intercostal muscle, and should not be confused with a pathologic process.
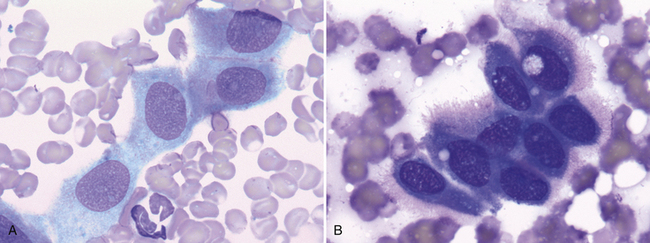
Figure 17-7 Visceral and parietal mesothelial cells may be incidentally aspirated when performing FNA of an intrathoracic lesion. A, Lung, FNA, dog. Small sheets of normal mesothelium consist of uniform, polygonal cells with light blue, finely granular cytoplasm and a central, round to oval nucleus. (Wright stain. Original magnification 1000×.) B, Lung, FNA, dog. Reactive mesotheliocytes often appear round to polygonal with dark blue cytoplasm that may have a peripheral, pink halo or “fringe.” (Wright stain. Original magnification 1000×.)
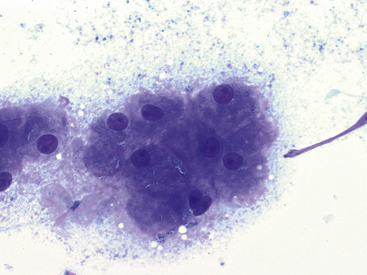
Figure 17-8 Lung FNA, dog.
The presence of hepatocytes is suggestive of inadvertent, trans-diaphragmatic aspiration of the liver and should not be confused with a pathologic process. (Wright stain. Original magnification 500×.)
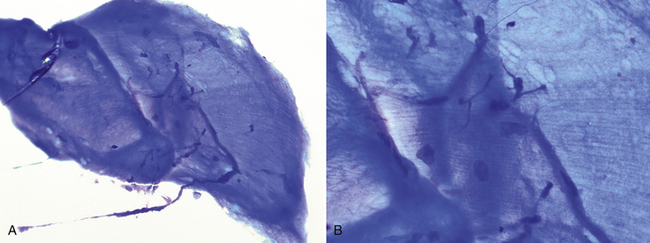
Figure 17-9 Lung FNA, skeletal muscle, dog.
Low magnification (A) (200×) and high magnification (B) (500×) images. (Wright stain.) Aggregates of well-differentiated striated skeletal myocytes often appear bright blue with compressed nuclei and visible striations. Most commonly, this is an incidental finding consistent with aspiration of intercostal muscle or the diaphragm.
Inflammation
Neutrophilic Inflammation
As in other tissues, increased proportions of neutrophils are associated with acute and chronic inflammation. Common causes of neutrophilic inflammation include bacterial infection, neoplasia, necrosis, and the presence of foreign material or other (nonbacterial) infectious agents. The cause of inflammation may or may not be evident cytologically, but care should be taken to look for intracellular bacterial organisms, neoplastic cells, necrotic debris, foreign material, and other infectious agents. Cytologically, necrotic debris appears as amorphous, bluish-gray material that may be present scattered throughout the smear or in dense aggregates (Figure 17-10). Foreign material may be of varying morphology; its presence may be suspected on the basis of the observation of unidentified, nonorganismal material that may be surrounded by dense aggregates of inflammatory cells. Cytologic findings associated with specific infectious and neoplastic lesions are discussed subsequently.
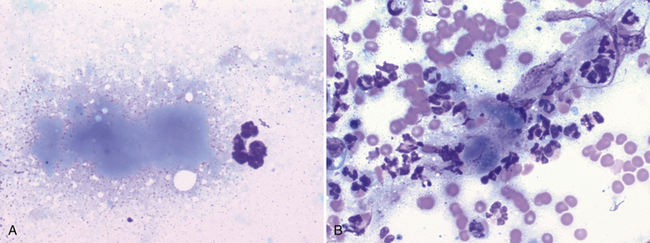
Figure 17-10 Lung FNA, dog.
Necrotic debris often appears as amorphous, bluish-gray material. In (A), a single small focus of necrotic debris is next to a neutrophil. (Wright stain. Original magnification 1000×). In (B), the necrotic debris is associated with neutrophilic inflammation and blood contamination. (Wright stain. Original magnification 1000×.)
Macrophagic, Granulomatous, and Mixed Inflammation
Lipid pneumonia is an uncommon disorder that has been observed in both dogs and cats.22,23 It is characterized by accumulations of highly vacuolated macrophages and, on occasion, fewer numbers of lymphocytes and neutrophils. Characteristics of granulomatous inflammation (e.g., multinucleate giant cells, epithelioid macrophages) may also be seen.
Eosinophilic Inflammation
When eosinophils are a prominent feature of the sample (>10% of all nucleated cells in the absence of peripheral eosinophilia), etiologies of eosinophilic inflammation should be considered. (Figure 17-11). Causes of eosinophilic inflammation include allergic or hypersensitivity disorders, parasite infestation, and some fungal, bacterial, and neoplastic diseases. It is not unusual to find low numbers of scattered mast cells in lesions with eosinophilic inflammation.
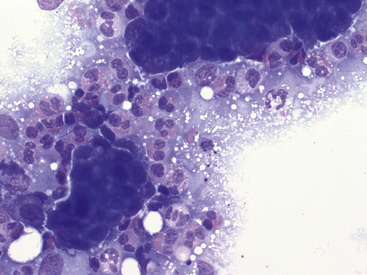
Figure 17-11 Lung FNA, dog.
Moderate numbers of eosinophils surround clusters of neoplastic epithelial cells in a dog with a carcinoma. (Wright stain. Original magnification 500×.)
Eosinophilic bronchopneumopathy, pulmonary infiltration with eosinophils (PIE), eosinophilic pneumonia (EP), and pulmonary eosinophilia (PE) have been used to describe various disorders characterized by eosinophilic infiltration of pulmonary parenchyma for which the etiology is not known. Inconsistent use of this terminology has led to confusion. Clercx and Peters used the term eosinophilic bronchopneumopathy (EBP) to describe eosinophilic infiltration that affects both the airway and pulmonary interstitium and suggested the term simple eosinophilic pneumonia (SEP) for cases (although not described in veterinary species) in which only the parenchyma is affected.24 EBP encompasses those lesions traditionally described as PIE or PE in the veterinary literature. EBP is associated with a bronchointerstitial radiographic pattern. A complete blood count (CBC) in these patients may or may not reveal peripheral eosinophilia.25 EBP is believed to be a hypersensitivity disorder, however, its cause has not been definitively determined.24 It is most often suspected when eosinophilic inflammation is observed on cytologic analysis of BAL fluid, however, identification of increased proportions of eosinophils (and exclusion of other causes of eosinophilic inflammation) on FNA samples of pulmonary parenchyma should prompt inclusion of EBP in the differential diagnosis.
Eosinophilic granulomatous pneumonia (EGP, also pulmonary eosinophilic granulomatosis) is a parenchymal disorder described in dogs, which is characterized by multiple pulmonary masses (granulomas), composed of eosinophils, macrophages, multinucleate giant cells, lymphocytes, and plasma cells.26,27 Although EGP is considered an idiopathic disorder, an association with dirofilariasis has been suggested.26 Hypersensitivity with associated granulomatous inflammation is a differential for this lesion.
Infectious Diseases
Bacterial Infection
Cytologic specimens that aid in the diagnosis of bacterial pneumonia are most commonly obtained via transtracheal washes or bronchoalveolar lavages (see Chapter 16). However, FNA of infected pulmonary tissue (diffuse disease or large focal lesions) may also be diagnostically valuable.28 A wide spectrum of bacterial organisms can cause pulmonary infections in the dog and cat.29 Extracellular bacteria may be true pathogens or contaminants. Observing bacteria within neutrophils, macrophages, or both suggests that they are true pathogens. Common pulmonary bacterial pathogens of the dog and cat may be rod shaped or coccoid. Bacterial morphology is not sufficient to identify its genus and species; culture, molecular diagnostics, or both are necessary to definitively identify the organism. However, few bacteria do exhibit morphology or staining characteristics that allow a presumptive etiologic diagnosis. For example, beaded, filamentous bacteria are consistent with an infection of Actinomyces spp., Nocardia spp., or (rarely) Fusobacterium spp. origin (Figure 17-12). Mycobacterium spp. is suspected when slender, nonstaining, rod-shaped elements are seen within macrophages or extracellularly (Figure 17-13). Mycobacterium spp. organisms do not stain with routine Romanowsky-type stains, but will stain reddish-pink with acid-fast stains.30
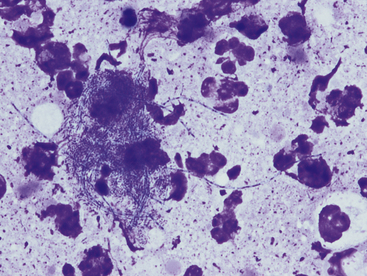
Figure 17-12 Thoracic fluid, dog, actinomycosis.
Several degenerate neutrophils surround a mat of bacteria. These beaded filamentous bacteria are consistent with Actinomyces, Nocardia, or Fusobacterium spp. (Wright stain. Original magnification 1000×.)
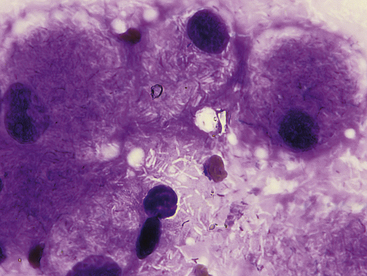
Figure 17-13 Skin, impression smear, cat, mycobacteriosis.
Epithelioid macrophages contain many negatively-stained rod-shaped bacteria consistent with Mycobacterium spp. These bacteria are commonly more visible when extracellular in a proteinaceous background. (Wright stain. Original magnification 1000×.)
Viral Infection
Viral infection is a rare cytologic diagnosis. Common viruses such as canine distemper virus and canine adenovirus have been associated with intracellular inclusion bodies seen histologically, but these inclusions are very rarely seen on cytologic specimens.31 Observation of secondary inflammation, bacterial infections, or both is more commonly found in cytologic specimens from virally infected animals.
Fungal Infection
Pulmonary parenchyma may be infected by many different mycotic organisms. When infections result in the formation of morphologically distinct yeast, the genus of the fungus may typically be definitively determined via cytology (Table 17-1; Figures 17-14, 17-15, 17-16, 17-17, and 17-18).32–36 In contrast, when intralesional hyphae are observed, the causative organism cannot be definitively determined by routine cytologic evaluation, as visually distinct characteristics are not commonly observed. A relatively common hyphae-forming fungal organism that has been diagnosed via FNA of the pulmonary parenchyma is Aspergillus spp. (Figure 17-19).37
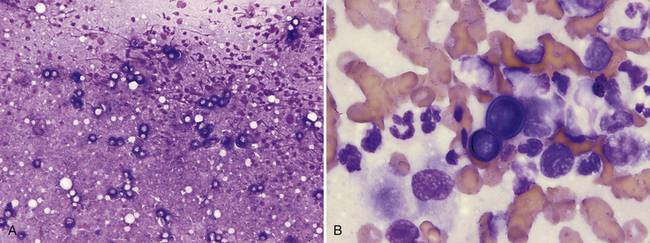
Figure 17-14 Lung, FNA, dog, blastomycosis.
A, Many pale-staining, thick-walled yeast can be seen at low-power magnification. (Wright stain. Original magnification 200×.) B, Basophilic, broad-based budding Blastomyces dermatitidis yeast with thick walls are surrounded by poorly preserved neutrophils and macrophages. (Wright stain. Original magnification 1000×.)
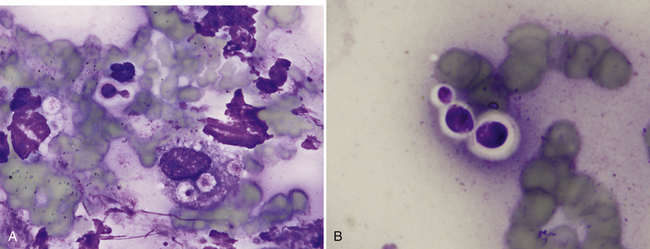
Figure 17-15 Nose, impression smear, cat, cryptococcosis.
A, Narrow-based budding Cryptococcus spp. yeast with a thick, nonstaining capsule are found extracellularly and surrounded by poorly preserved neutrophils, a macrophage, and cellular debris. Also present within the macrophage are three, poorly-stained acapsular yeast. B, Three extracellular Cryptococcus spp. yeast of variable size exhibit a thick, nonstaining capsule. (Wright stain. Original magnification 1000×.)
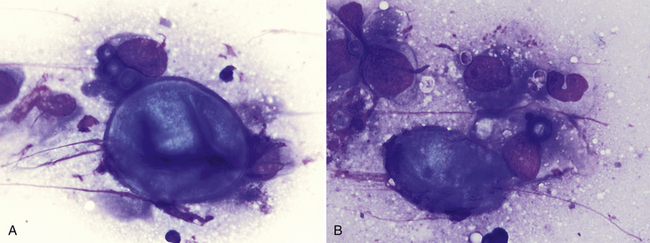
Figure 17-16 Skin, FNA, cat, coccidioidomycosis.
A, One large extracellular spherule (measuring 35 µm in diameter) with a thick, well-demarcated wall is adjacent to a macrophage containing three smaller spherules. B, One large extracellular, partially folded spherule is adjacent to a macrophage containing one small spherule. Few small extracellular endospores (3-5 µm) are also present. (Wright stain. Original magnification 1000×.) (Glass slide courtesy of Sharon Dial).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree