CHAPTER 37 The Liver, Biliary Tract, and Exocrine Pancreas
Development of the Hepatobiliary System and Pancreas
Development of the Hepatic Circulation
Metabolic Functions
Biochemical indicators of hepatic disorders
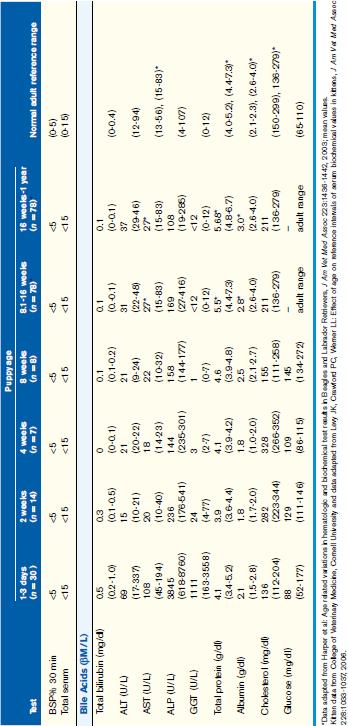
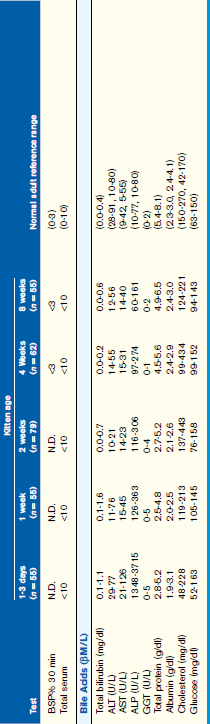
Normal values for routinely used biochemical indicators reflecting the status of the hepatobiliary system in newborn and growing puppies and kittens are given in Table 37-1.
Blood glucose
Although glucose regulation improves with age, puppies and kittens up to 4 months of age should be considered predisposed to hypoglycemia when anorexic or dehydrated. Conditions and clinical signs associated with neonatal/pediatric hypoglycemia are provided in Box 37-1. It is notable that severity of clinical signs increases with age such that hypoglycemia is more easily recognized in older animals. Thus maintaining a high index of suspicion for hypoglycemia is essential to achieve early diagnosis in a neonate. Treatment is aimed at achieving euglycemia, normalizing body temperature and hydration status, avoiding stress, and eliminating underlying causal factors.
Liver enzyme activity
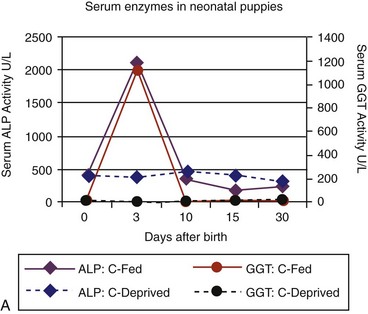
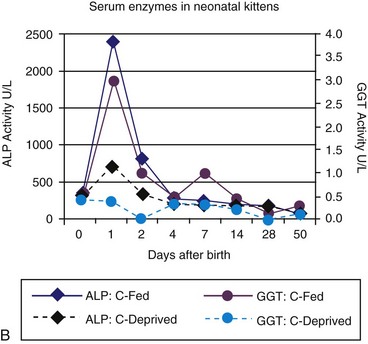
Age-appropriate reference intervals for serum liver enzyme activity are essential for interpreting laboratory data in neonatal puppies and kittens. Differences in serum enzyme activities between neonates and adults reflect physiologic adaptations during the transition from fetal and neonatal life stages, trauma associated with birthing, colostrum ingestion, maturation of metabolic pathways, growth effects, differences in volume of distribution and body composition, and nutrition. Activity of serum alkaline phosphatase (ALP), aspartate aminotransferase (AST), creatine kinase (CK), and lactate dehydrogenase (LDH) usually increase greatly during the first 24 hours of life. In kittens, ALP, CK, and LDH activity exceeds adult values through 8 weeks of age, whereas AST increases only transiently after birth. The early increases in AST, CK, and LDH likely reflect muscle trauma associated with birthing, whereas ALP activity reflects bone isoenzyme associated with bone growth. Enteric absorption of colostral macromolecules during the first day of life causes a substantial increase in ALP in puppies and kittens, and of gamma-glutamyltransferase (GGT) in puppies (Figure 37-1, A and B). This phenomenon is not unique to dogs and cats as it has also been documented in neonatal calves, lambs, pigs, foals, and human infants. Studies also have confirmed significant differences in ALP activities develop between colostrum-deprived and suckling pups and kittens within 24 hours of birth, with a similar change in GGT also observed in puppies. These differences are short lived, resolving within the first 2 weeks, but can be used as a surrogate marker of effective colostrum ingestion. Studies have confirmed that colostrum contains substantially higher GGT and ALP activity than that resident in the serum of the respective dam or queen. For example, colostral or milk GGT in bitches is 100-fold and ALP is tenfold greater than sera until day 10. However, by day 30, GGT and ALP activity in milk is significantly lower than before suckling had commenced. Although a marked influence of colostrum on serum ALP activity in neonatal kittens also occurs, the effect on GGT is modest compared with that in neonatal puppies. Sustained increases (first 6 to 12 months of life) in serum ALP activity in puppies and kittens (maximally threefold more than high normal adult reference values) reflect the bone ALP isoenzyme derived from osteoblast activity.
Albumin, globulins, coagulation factors, and protein C
Synthesis of albumin, many globulins, most coagulation, and many anticoagulant factors depends on the liver. Total protein and albumin concentrations in young dogs up to 4 weeks of age are below normal limits for adults, whereas protein concentrations in young cats are more variable (see Table 37-1). By 8 weeks of age, puppies have normal adult albumin concentrations, whereas total globulin values increase with age, reflecting cumulative antigenic challenge. Although coagulation assessments are uncommonly completed in neonatal puppies and kittens, limited observations suggest that values fall within the normal adult ranges for prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen in animals as young as 8 weeks of age. Protein C, recently shown to discriminate normal puppies from puppies with PSVA, is influenced by adequacy of portal venous perfusion. Although liver failure and severe enteric protein loss also can compromise protein C activity, this anticoagulant factor is useful for differentiating PSVA from microvascular dysplasia (MVD) in young dogs.
Cholesterol
Serum cholesterol concentration can reflect hepatic functional and circulatory disturbances. Because cholesterol is synthesized in the liver, synthetic failure can cause marked hypocholesterolemia. Portosystemic shunting, either congenital or acquired, also causes mild to marked hypocholesterolemia. Because cholesterol is excreted into the biliary tree in bile, cholestasis can increase in cholesterol concentrations. A mild to moderate increase in cholesterol is apparent with acute obstructive jaundice and in some animals with acute severe hepatic inflammation (lacking synthetic failure). In 1- to 3-day-old puppies but not kittens, mild hypocholesterolemia is common (see Table 37-1). In puppies and kittens older than 2 to 4 weeks of age, serum cholesterol concentrations are within the adult normal range.
Hepatic mineral storage
Age-related variations in hepatic concentrations of iron, copper, zinc, and selenium (per gram of dry liver weight) have been reported for Beagle dogs from 8 to 193 days of age (Table 37-2). A decrease in hepatic iron concentrations during the first 20 days after birth likely reflects mobilization of iron for hemoglobin synthesis in bone marrow, the relative iron deficiency of a milk diet, and decrease in hepatic extramedullary hematopoiesis. In many species, hepatic copper concentrations are higher in pediatric individuals relative to adults. In dogs, hepatic copper concentrations change little with advancing age unless challenged with a copper-rich diet. In dogs with copper-associated hepatopathy (primary metabolic or copper transport disorder, dietary copper loading, cholestatic liver injury), hepatic copper concentrations significantly increase over time (see later discussion on Copper Storage Hepatopathy).
TABLE 37-2 Hepatic mineral concentrations in Beagles (mean ± standard deviation; µg/g dry weight)
| Mineral content | 8 to 40 days of age (n = 10) | >40 days of age (n = 20) |
|---|---|---|
| Iron | 1025 ± 882 | 585 ± 258 |
| Copper | 285 ± 75 | 304 ± 90 |
| Zinc | 225 ± 88 | 143 ± 30 |
| Selenium | 2.5 ± 0.4 | 1.9 ± 0.4 |
Summarized from Keen CL, Lonnerdal B, Fisher GL: Age-related variations in hepatic iron, copper, zinc, and selenium concentrations in beagles, Am J Vet Res 42:1884, 1981.
Hepatobiliary Disorders of the Young Dog and Cat
Survey of young dogs and cats (n = 444; puppies, n = 312 and kittens, n = 132) with liver tissue examined histologically (biopsy or necropsy) over a 12-year interval in the author’s hospital is detailed in Table 37-3. Various histologic and definitive diagnoses are represented. Hepatic necrosis, hepatic congestion, and hepatic lipidosis were the three most common histologic features. Hepatic congestion is of uncertain significance as this may represent a terminal or death-related change.
TABLE 37-3 Hepatobiliary disorders in puppies and kittens less than 4 months of age: gross and histopathologic tissue evaluations
| Disorder | Puppies (n = 312) | Kittens (n = 132) |
|---|---|---|
| Infectious hepatopathies | 68 | 18 |
| Parvovirus | 30 | 0 |
| Herpesvirus | 7 | 0 |
| Canine distemper | 11 | — |
| Infectious canine hepatitis | 4 | — |
| Feline infectious peritonitis | — | 17 |
| Parasite migration | 4 | 0 |
| Hepatic abscessation | 12 | 1 |
| Severe hepatic necrosis | 54 | 41 |
| Hepatic congestion | 63 | 25 |
| Hepatic lipidosis | 36 | 16 |
| Extramedullary hematopoiesis | 39 | 10 |
| Cholangitis | 9 | 10 |
| Trauma (hematoma, laceration) | 10 | 6 |
| Nonspecific hepatitis | 14 | 3 |
| Portosystemic vascular anomaly | 1 | 11 |
| Hepatic atrophy | 3 | 2 |
| Vasculitis | 1 | 0 |
| Lymphosarcoma | 1 | 0 |
| Diaphragmatic hernia (liver involvement) | 1 | 0 |
Congenital Anatomic Malformations
Gallbladder
Congenital malformations of the gallbladder are most common in the cat. Congenital division of the gallbladder is most common and is also referred to as an accessory, cleft, diverticular, or bilobed gallbladder (Figure 37-2). These malformations involve the initial subdivision of the primary cystic diverticulum or a bud from the neck of the embryonic gallbladder. Although such malformations do not cause clinical illness, they can cause confusion when recognized during abdominal ultrasonography as they may be mistaken for a cyst.
Cystic hepatobiliary lesions
Cats with cystic liver lesions should be DNA tested for the renal polycystic gene mutation if they are intended for breeding. It remains unclarified if there are variants of this disorder associated with primary liver involvement. DNA testing is done using a cheek swab kit (available from felinegenome@ucdavis.edu). Treatment is usually not indicated for congenital cystic liver lesions unless a large cyst causes abdominal discomfort or fluid accumulation causes pressure effects on adjacent organs or tissues. Periodic aspiration of large problematic cysts has been used to manage some patients. Other alternatives include partial cyst wall resection, entire cyst excision, or removal of an involved liver lobe. In some Persian cats, the polycystic renal or hepatic involvement is recognized during the first few months of life. In some of these, polycystic kidney disease is rapidly lethal. In others, the disorder is mild, does not cause overt signs, and is recognized incidentally later in life.
Common Vascular Malformations Involving the Liver
Portosystemic vascular anomaly
Physical findings usually include a small body stature and unthrifty appearance. Abdominal palpation often discloses prominent kidneys. Large kidney size may relate to increased GFR, renal gluconeogenesis, and other designated metabolic functions not normally conducted by the kidney, as well as renal cell hypertrophy induced by high ammonia concentrations. Rarely cystic calculi are palpated. A unique copper-colored iris is observed in non–blue-eyed cats (Figure 37-3); this color is similar to the eye color in Persians and thus must not be interpreted out of context.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree