Chapter 20
The Liver
Cytologic examination of samples from the hepatobiliary system complements other diagnostic procedures and, in some cases, provides the specific, definitive diagnosis. Specimens for cytologic examination may be collected by percutaneous fine-needle aspiration (FNA), percutaneous needle core biopsy, or surgical biopsy. Cytologic examination of material collected by FNA or fine-needle biopsy (FNB), which are relatively inexpensive and safe procedures, determine whether additional sampling or exploratory surgery is warranted. Collection and examination of cytologic samples are most commonly performed on patients with nodular lesions, abnormal echogenicity, or generalized or lobar liver enlargement. It may also be used prior to therapy in patients with persistently increased liver enzymes or to stage certain neoplastic conditions such as lymphoma and mast cell neoplasia. The increasing availability of imaging procedures such as ultrasonography and CT, to direct specimen collection via FNA and FNB has increased the accuracy of sampling nodular lesions, thereby increasing the diagnostic acuity of liver cytology. Studies reported in the literature have shown that the accuracy of cytologic interpretations is greatest for diffuse hepatic disease and neoplasia, and least sensitive for subtle inflammatory conditions.1,2
Sampling Techniques
Various techniques for percutaneous sampling of the hepatobiliary system have been described.3–6 Complications following these procedures are rare and include hemorrhage and potential seeding of needle tracts with neoplastic cells. As compromised hemostasis is a life-threatening concern in animals with liver disease, the risk for prolonged or excessive bleeding should be assessed, particularly in cats with prolonged anorexia and in severely ill patients.4,7 A thorough history taking should include querying the owner about any prior bleeding episodes or current treatment with prescribed or over-the-counter drugs such as aspirin or ibuprofen, which may alter normal coagulation. This is followed by a complete physical examination and laboratory testing to evaluate platelet number (platelet count or estimate from a peripheral blood smear), buccal mucosal bleeding time (BMBT), and coagulation assays (prothrombin time [PT], activated partial thromboplastin time [APTT], and protein-induced vitamin K absence). It must be kept in mind that normal coagulation testing profiles do not exclude potential bleeding episodes.8 It is pragmatic to avoid aspirating the highly vascular liver if severe coagulation abnormalities, clinical signs of hemorrhage elsewhere in the body, or both exist. If a liver aspirate is deemed an acceptable risk in a patient with mildly prolonged coagulation tests, it is prudent to perform it early in the day so that the hematocrit can be followed and interventional treatments performed if excess bleeding is detected.
FNA and FNB are often performed using ultrasound guidance, especially when nodular lesions are detected. Sensitive imaging procedures such as ultrasonography facilitate accurate sampling of the liver, including sites with normal and abnormal echogenecity.3,4 Blind sampling may be done with the patient in dorsal or right lateral recumbency but also in the standing position; chemical restraint may be unnecessary for some patients. With the patient in dorsal recumbency, a blind biopsy is performed by inserting (at a 30- to 45-degree angle to the skin in dogs, and almost vertically in cats) midway between the left costal arch and the end of the xiphoid process.2 If the animal is standing or lying on the right side, the collection site is found by percussing the intercostal spaces to locate the liver beneath the rib cage. The next intercostal space caudal to the space where the sound of percussion changes from resonant to dull is usually a satisfactory site for aspiration. The collection site may be determined by palpation if an enlarged liver extends beyond the rib cage in which case the site would be just caudal to the costal arch.5 In some cases, the clinician may sample on the right side at the tenth intercostal space at the level of the rib-cartilage junction.5
Cells from the hepatobiliary system may be aspirated using a 21- to 22-gauge, 1 to 2½-inch hypodermic needle and a 6- or 12-milliliter (mL) syringe. Alternatively, a 22-gauge spinal needle with a stylet may be used. The stylet prevents obstruction of the needle as it penetrates the body wall. With the needle in the liver, samples are aspirated by rapidly withdrawing the plunger of the syringe to the 5- or 6-mL mark. The suction is gently released before the needle is withdrawn. The specimen will consist of small pieces of parenchymal tissue mixed with fresh blood. Once the needle is withdrawn, the syringe should be detached and filled with 1 to 2 mL of air. After reattaching the syringe to the needle, a small drop of sample (6 to 8 millimeter [mm] diameter) is expelled onto each of several glass slides. The drops are placed near the center of the slide and spread gently into a smear by the squash method (see Chapter 1).
The liver may also be sampled by a nonaspiration technique, using a 26-gauge needle only, as described in Chapter 1. This method may reduce the amount of blood contamination but may result in exfoliation of fewer hepatobiliary cells.5
Major Cytologic Classifications of Liver Processes
Normal Liver
To accurately interpret hepatic aspirates, normal hepatocytes and biliary epithelium as well as normal parenchymal features should be recognized. Aspirates and impressions from normal livers consist largely of hepatocytes and variable amounts of peripheral blood. Hepatocytes exfoliate readily and are distributed in the smear as single cells, as sheets, and in cords and clusters. Normal hepatocytes are fairly uniform, large, round, or slightly oval to polyhedral cells that have round, centrally placed nuclei, with stippled coarse chromatin and a single large prominent nucleolus (Figure 20-1). Their abundant cytoplasm contains many ribosomes, which stain blue and other organelles that are unstained or slightly pink, giving the overall color of the cytoplasm in most normal hepatocytes a grainy light blue or lavender, with faint pink cytoplasmic granules. Hepatocytes in large clusters, which resist flattening, have a deeper blue cytoplasm compared with single cells or cells in flattened sheets. In older animals, pigment granules may be seen in the cytoplasm of the hepatocytes (see the section titled “Pigments” below). Nuclei typically are centrally located, uniform in size, and have coarsely reticular chromatin. A single nucleolus is often visible; however, in some intact cells, this may be obscured by heterochromatin. The nucleoli are more easily seen in the nuclei of ruptured or understained cells. Low numbers of binucleate hepatocytes are commonly present in liver aspirates from older dogs, and few rectangular crystalline intranuclear inclusions may also be noted (Figure 20-2). The pathologic significance of these inclusions is not clear; however, they are felt to be increased in cases of chronic liver insult or disease.9 In some cases of canine herpes virus -1 or adenovirus (infectious canine hepatitis), viral intranuclear inclusions are identified and must be differentiated from cytoplasmic invaginations into the nucleus.9
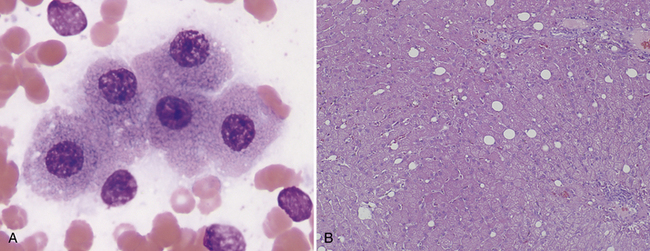
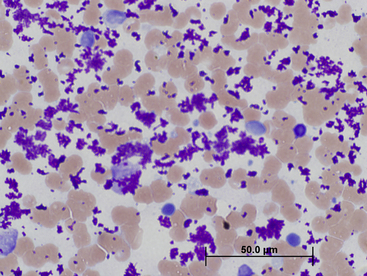
Figure 20-1 A, Cytologically normal hepatocytes from a dog. These cells may be from a normal liver, or an unaffected area of a diseased liver. Normal-appearing hepatocytes may be seen in areas of hyperplastic nodules, hepatic adenoma, or well-differentiated hepatic carcinoma. (Wright stain. Original magnification 400×.) B, Normal canine liver histology. (H&E stain. Original magnification 200×.)
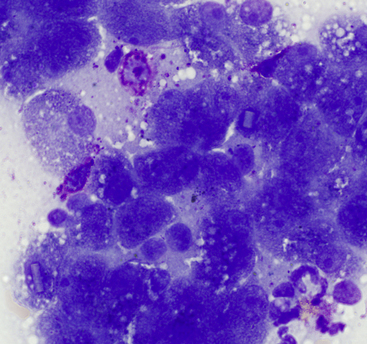
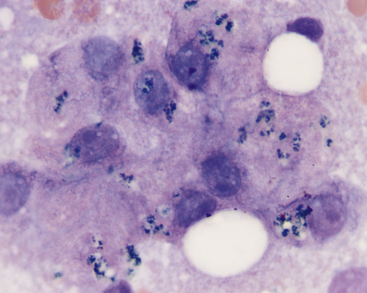
Figure 20-2 Liver aspirate from a 12-year-old Labrador Retriever presented for vomiting.
Hyperplastic hepatocytes with occasional resident well-granulated mast cells are present (also note the intranuclear crystalline inclusions). (Wright stain. Original magnification 1000×.)
Several other cell types are commonly encountered in normal liver aspirates. Biliary tract epithelial cells appear as uniform cuboidal to short columnar cells in small clusters or flat sheets (Figure 20-3). Rarely, distinct tubular arrangements may be seen. Biliary cells have a high nucleus-to-cytoplasm (N:C) ratio; have round, central nuclei with dense smooth chromatin; and scant pale blue cytoplasm, which distinguishes them from noticeably larger hepatocytes. Mesothelial cells may be difficult to distinguish from biliary epithelial cells but are generally larger, polygonal to spindloid, and found in flat sheets with a mosaic pattern (Figure 20-4). Occasional samples may include extrahepatic tissue such as adipocytes, lipid, or both when mesenteric fat is inadvertently aspirated. Most cytologic preparations of liver aspirates will contain some peripheral blood, and normal blood leukocytes are expected in numbers consistent with the complete blood count (CBC). Mast cells are normal resident perivascular cells, and therefore, with the highly vascular nature of the liver, few are anticipated in normal dog and cat liver aspirates (see Figure 20-2).
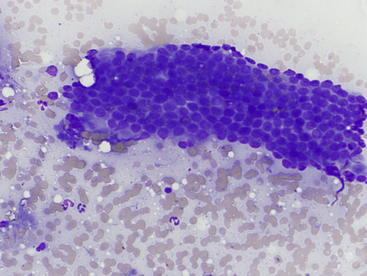
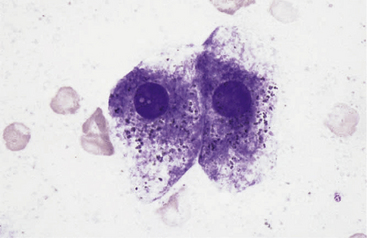
Figure 20-3 Normal biliary tract epithelial cells.
Note the flat sheet of cuboidal to low columnar cells in a tubular structure and low numbers of neutrophils. (Wright stain. Original magnification 500×.)
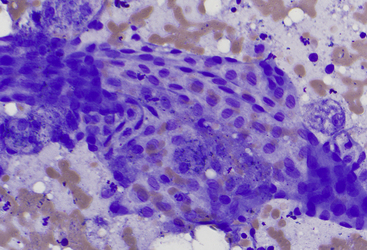
Figure 20-4 Liver aspirate from a dog.
Note the regular angular cellular characteristics of exfoliated mesothelium and few normal hepatocytes. (Wright stain. Original magnification 500×.)
Resident hepatic macrophages (Kupffer cells) may be aspirated from normal liver, and rare lipid-laden Ito cells may be identified in some aspirates. In cats, low numbers of lymphocytes may also be seen and are of equivocal significance because a few lymphocytes may be found in the portal triads in healthy animals.10
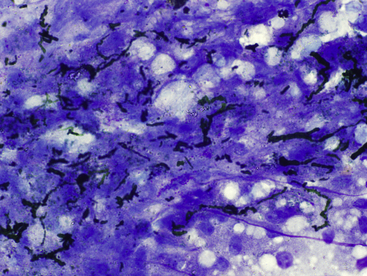
A common and significant artifact of ultrasound-guided liver biopsies is the inadvertent contamination with ultrasound gel. After staining with Romanowsky-type stains, this material often appears globular and dark magenta to metachromatic and may be mistaken for mast cell granules or large granules in lymphocytes. The presence of this material in samples may vary from slight interference of staining and appreciation of cellular detail to completely obscuring the sample (Figure 20-5). Ultrasound gel has been reported to cause cellular disruption in human cytology samples.11 In the experience of the chapter authors, this also occurs in veterinary cytology specimens; however, this has not been documented in domestic animal cytology preparations.
Pigments
Several different pigments may be seen within hepatocytes in cytologic preparations and may be a normal finding or associated with extrahepatic or intrahepatic disease. The most common pigments include bile pigment, hemosiderin, lipofuscin, and copper. Specific staining is often required for differentiation, as in a recent study, it was determined that the blue-green pigment often interpreted as bile is, in fact, lipofuscin.12 Differentiating the pigments solely on the basis of routine staining with Romanowsky stains such as Wright-Giemsa or modified Wright-Giemsa (Diff-Quik, Leukostat, or Quik-Dip) may be difficult, and special stains may be employed for specific identification, when required.
Bile Pigment
Bile accumulation within hepatocytes is recognized as variably sized, dark-green to black granules in cytologic preparations and yellow to green-brown on standard hematoxylin and eosin (H&E) histologic sections (Figure 20-6). Abundant intracytoplasmic bile pigment is suggestive of cholestasis and may precede hyperbilirubinemia and clinical icterus. More significant accumulations of bile result in bile-filled cannalicular plugs, which are easily recognized on cytologic preparations (Figure 20-7). In some cases, the cause of cholestasis may be determined by an accompanying inflammatory or neoplastic infiltrate; in other cases, the cause is not readily identified.
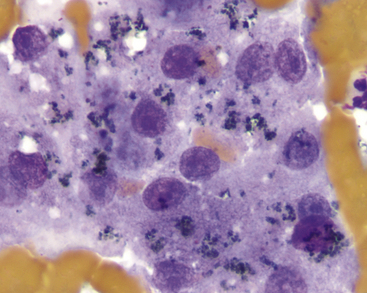
Figure 20-6 Liver aspirate from a dog with elevated γ-glutamyl transferase (GGT).
Dark-green to black granular pigment suggests bile pigment, which is readily observed in cholestasis. (Wright stain. Original magnification 400×.)
Lipofuscin
Lipofuscin is a “wear and tear” pigment, composed of accumulated indigestible cellular residue within autophagolysosomes. Large amounts may be seen in hepatocytes from older cats and dogs. This is a normal feature of aging and does not represent a pathologic process. Granules of lipofuscin are similar in size to bile pigment and hemosiderin and range from brownish to blue-green (Figure 20-8). Lipofuscin is yellow-brown on standard H&E staining of histologic sections and often centrilobular in location. On the basis of one study, lipofuscin is the pigment most commonly observed within canine hepatocytes and correlates with a lack of supportive evidence of cholestasis such as hyperbilirubinemia, bile cannalicular plugs, or casts.12 Lipofuscin granules specifically stain red with a modified Ziehl-Neelsen stain or Luxol fast blue.
Hemosiderin
Hemosiderin is a globular, insoluble, iron-containing material, which appears golden-brown in hepatocytes (Figure 20-9) with Romanowsky-type stains and dark-blue with Prussian Blue stain. Histologically, with H&E staining, iron appears golden brown and refractile. In many cases, increased hemosiderin in hepatocytes is associated with increased iron turnover caused by hemolytic anemia or ineffective erythropoiesis (such as immune-mediated nonregenerative anemia). Iron also may accumulate because of decreased iron utilization associated with severely decreased erythropoiesis (pure red blood cell [RBC] aplasia, aplastic anemia). Repeated blood transfusions or administration of iron-containing compounds (especially by injection) also may increase hepatic iron content. In macrophages, hemosiderin may vary from gold to brown-black, depending on the amount within the cell. Macrophages containing phagocytized RBCs, hemosiderin, or both are concomitant findings in many patients with hemolytic or hemophagocytic disease.
Copper
When present in large amounts, copper may be visible in hepatocytes as coarsely clumped, refractile, pale blue-green cytoplasmic granules (Figure 20-10, A). Liver smears may be stained with rubeanic acid to confirm the presence of copper (see Figure 20-10, B). Large amounts of copper are often associated with a primary copper-accumulation hepatopathy.9 Small amounts of stainable copper may be seen secondary to prolonged cholestatic liver disease and in cases of chronic hepatitis. Primary copper hepatopathy has been reported as a familial condition in Bedlington Terriers because of a genetic mutation in the COMM-D gene. This gene produces a defective protein involved in copper transport; thus, excessive copper accumulation occurs in the liver. Copper accumulation also seems to be familial in West Highland White Terriers, Skye Terriers, Doberman Pinschers, Labrador Retrievers, and Dalmations.5,9,13–15 Copper-associated hepatopathy in a young Siamese cat has also been reported.16 Excess copper in hepatocytes leads to injury or necrosis with inflammation.
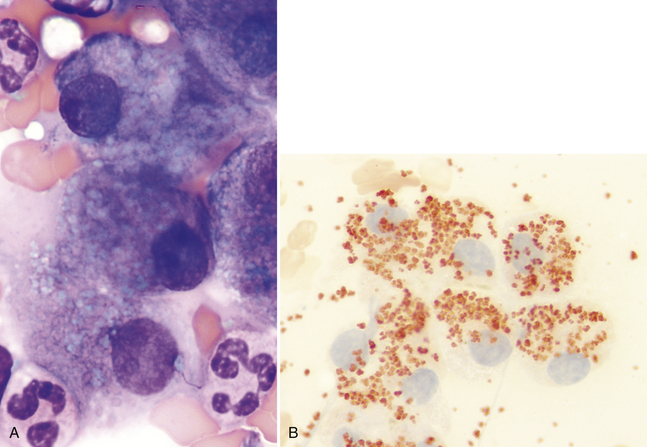
Figure 20-10 A, Liver aspirate from a 3-year-old Bedlington Terrier with copper-associated hepatopathy. Copper in hepatocytes appears as coarsely clumped, refractile, pale blue-green cytoplasmic granules. (Wright stain. Original magnification 400×.) B, Rubeanic acid highlights excess cytoplasmic copper in canine hepatocytes.(Rubeanic stain. Original magnification 400×.) (A, Case material from Dr. Michael Scott, 1991, ASVCP slide review session.)
Nonneoplastic Disorders and Conditions
Degenerative Conditions
Hepatocellular vacuolar degeneration is a common yet nonspecific cytologic finding, and it results from the intracellular deposition of glycogen, lipid, or water and is recognized as either discrete, round vacuoles or poorly circumscribed, pale, feathery areas within the cytoplasm (Figure 20-11 and Figure 20-12). Hepatocellular steatosis may be seen as macrovesicular (vacuoles larger than the nucleus) or microvesicular (vacuoles smaller than the nucleus) inclusions. Microvesicular lipidosis is seen with more severe hepatocellular dysfunction and is correlated with lipid accumulation in mitochondria. 5 Discrete round vacuoles indicate lipid, whereas the less defined areas of feathery change or rarefaction often represent increased glycogen or water (hydropic degeneration) resulting from hypoxemia, cholestasis, or toxin exposure. If needed, lipid may be confirmed by staining an unfixed cytology smear with Sudan III; and glycogen may be confirmed by periodic acid-Schiff (PAS) staining. The most common vacuolar change recognized cytologically is hepatic lipidosis in feline samples and glucocorticoid-induced hepatopathy in canine liver aspirates.
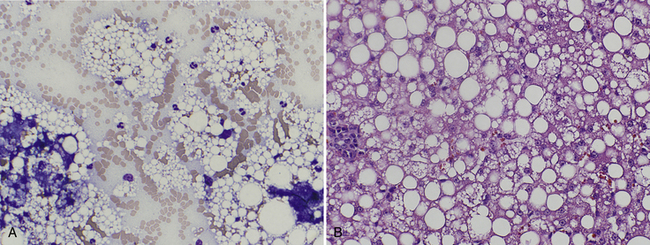
Figure 20-11 A, Hepatocytes from a 4-year-old cat with pancreatitis and hepatomegaly. Note the cytoplasmic hepatocellular vacuolar changes (demarcated, large and small discrete clear lipid – filled vacuoles) and increased neutrophils. Similar clear vacuoles are seen in the background released from ruptured hepatocytes. The cytologic diagnosis is lipidosis with suppurative inflammation (secondary lipidosis). (Wright stain. Original magnification 1000×.) B, Follow-up histologic section (liver biopsy taken two weeks after cytology due to persistently elevated alanine persistently elevated alanine aminotransferase) from the same patient with the histologic diagnosis of feline hepatic lipidosis (H&E stain. Original magnification 400×.)
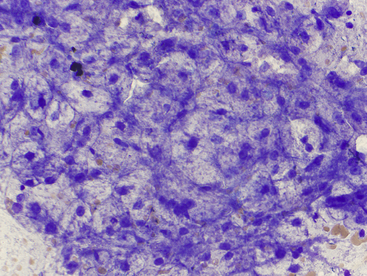
Figure 20-12 Hepatocytes from a 12-year-old dog with increased liver enzyme activity treated for 8 months with corticosteroids for a persistent cough.
Note the feathery rarefaction of the cytoplasm (nondiscrete vacuolization), which suggests glycogen accumulation associated with glucocorticoid effects, ischemic damage, or hepatoxin exposure. The diagnosis, in this case, was hyperadrenocortism and hypothyroidism. (Wright stain. Original magnification 500×.)
In cats, hepatomegaly caused by the accumulation of fat in the liver is recognized as the syndrome of feline hepatic lipidosis (steatosis). In this condition, often a combination of macrovesicular and microvesicular vacuole accumulation occurs. Development of this disorder generally is triggered by a period of anorexia causing a catabolic state.17 Primary lipidosis may be a result of inadequate food from owner-directed rapid weight loss, unacceptable food change, unintended food withdrawal, or stress. Secondary hepatic lipidosis may be affiliated with underlying systemic disease (lymphoma, pancreatitis, cholangiohepatitis, etc.).5 Affected cats are often icteric and have enlarged livers caused by extensive, diffuse accumulation of lipid within hepatocytes. Most cats with hepatic lipidosis have increased serum alkaline phosphatase activity and hyperbilirubinemia with no or only a slight increase in γ-glutamyltransferase activity. Hepatocytes from cats with hepatic lipidosis will contain round, sharply delineated empty vacuoles with a background punctuated with similar vacuolation. Most hepatocytes are distended by multiple vacuoles of various sizes that often peripheralize the nucleus and other organelles, leaving only a thin rim of visible cytoplasm. It is of utmost importance that the cytologist recognizes that feline hepatic steatosis may be primary or secondary and is therefore obligated to perform a thorough search for inflammation, infection, or neoplasia. Inherited lysosomal storage disease may produce similar vacuolar changes, but these conditions are very rare and more likely to cause recognizable changes in younger animals.
Hepatomegaly caused by lipid accumulation in hepatocytes is uncommon in dogs. Mild to moderate lipid vacuolization may develop secondary to various metabolic disorders in dogs and cats, especially diabetes mellitus. Severe hepatic lipidosis (microvesicular) with hypoglycemia may occur subsequent to anorexia in puppies of toy dog breeds.18 Dogs with aflatoxicosis develop severe hepatic lipidosis, in which liver aspirates resemble those from cats with feline hepatic lipidosis.19
In dogs, high plasma concentrations of glucocorticoid hormones (endogenous or exogenous) produce recognizable biochemical changes (increased serum alkaline phosphatase activity without hyperbilirubinemia) and cytomorphologic changes (several-fold enlargement of hepatocytes with vacuolar changes). Canine vacuolar hepatopathy of this type may be attributed to hyperadrenocorticism (HAC) or exogenous corticosteroid therapy, or “steroid hepatopathy” (see Figure 20-12). These patients generally have characteristic accompanying clinical signs (polyuria, polydipsia, polyphagia) with hepatomegaly. Excess glycogen and water cause the cytoplasm of these hepatocytes to swell, displacing the blue-staining, ribonucleic acid (RNA)–containing organelles. Aspirates will contain a mixture of swollen affected hepatocytes and intact normal hepatocytes.
Similar, nonspecific vacuolar changes and increased alkaline phosphatase activity, secondary to a variety of conditions (e.g., neoplasia, congenital or acquired hepatobiliary disease, nodular hyperplasia, GI disease, stress), are common findings in dogs.20
Irreversible cellular injury leading to death and necrosis of hepatocytes may occur from a variety of toxins (e.g., aflatoxins, cycad toxicity, wild mushrooms), drugs (e.g., tetracycline and diazepam in cats, rimadyl and xylitol in dogs), immune-mediated disease, metabolic disturbances, ischemia, neoplasia, and infectious agents. Cell death may occur via two pathways: apoptosis and necrosis. The two are largely distinct pathways, with apoptosis being “programmed cell death” and necrosis reflecting a pathologic condition. Apoptosis is important in the development and homeostasis of renewable cell populations. Apoptotic cells have pyknotic or karyorrhectic nuclei but intact cell membranes and cytoplasmic features. Hepatocellular necrosis involves altered cell membrane permeability and cellular swelling and is recognized as pink cytoplasm because of loss of RNA and denaturation of cytoplasmic proteins (Figure 20-13). Nuclei are condensed or disrupted or not discernible.
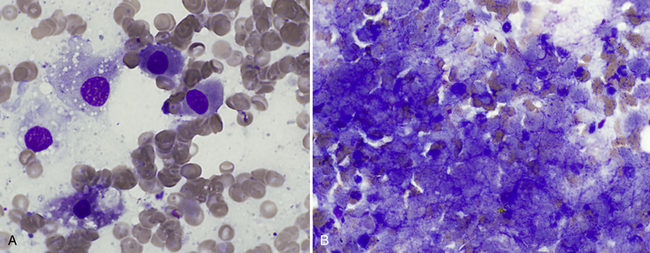
Figure 20-13 Hepatic necrosis in a liver aspirate from a young dog after eating wild mushrooms.
A, Early stage typified by single cell eosinophilic cytoplasm and a pyknotic nucleus. (Wright stain. Original magnification 1000×.) B, Later stage necrosis with large amounts of amorphous basophilic material resembling blurred cytoplasmic membranes and nuclei. (Wright stain. Original magnification 1000×.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree