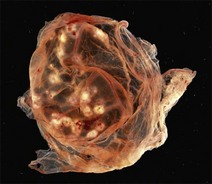
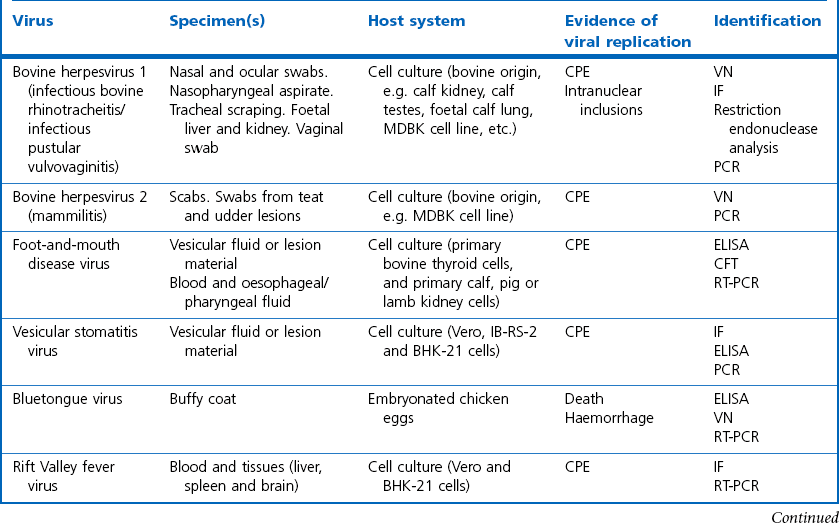
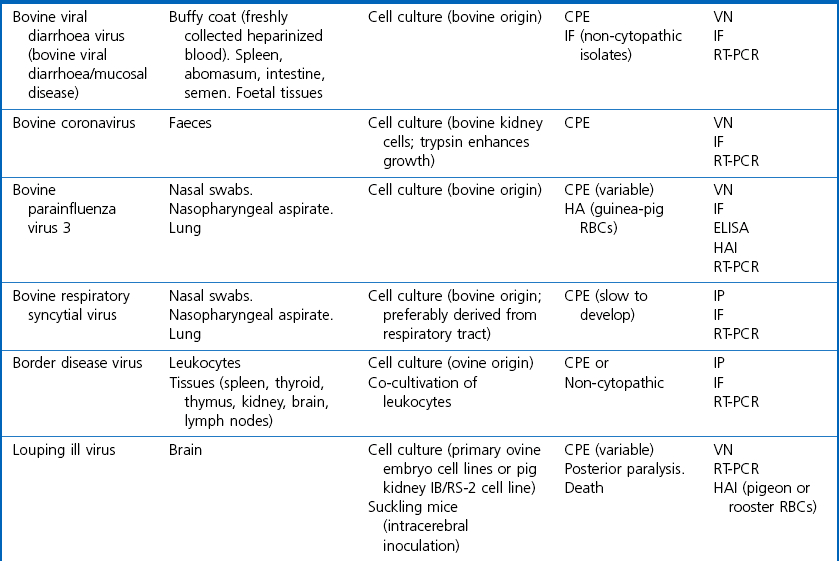
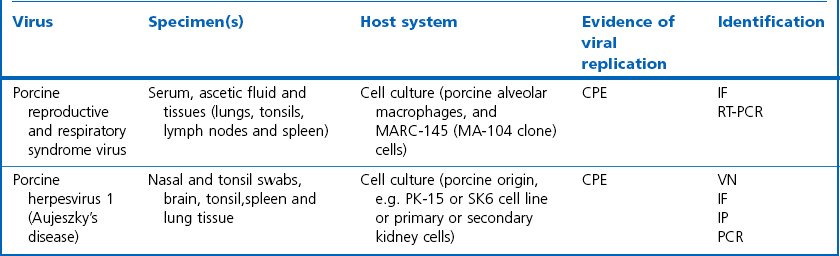
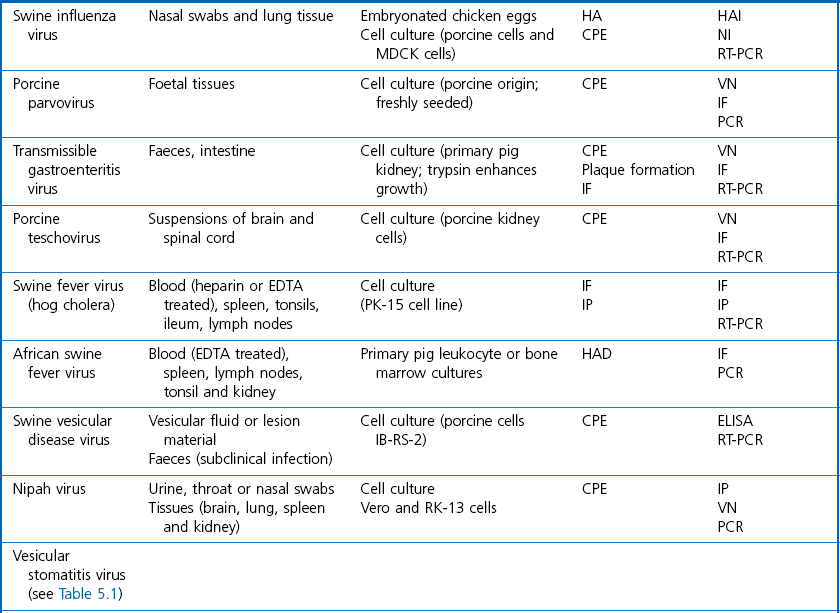
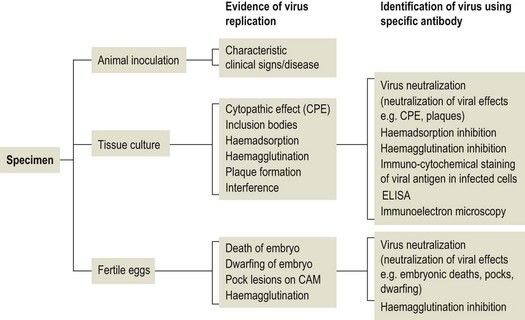
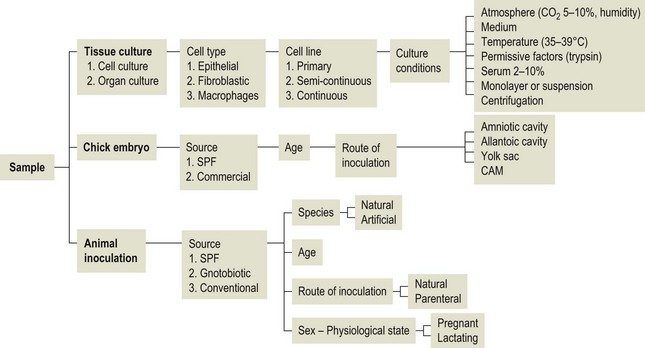
Chapter 5 Virus isolation is traditionally the definitive criterion against which other diagnostic methods are assessed. Viruses are obligatory intracellular parasites and can only be isolated in living systems. Isolation of a virus may be carried out in cell monolayers, fertile eggs or laboratory animals (Fig. 5.1). Thankfully, the use of laboratory animals has largely been supplanted. The vast majority of virus isolation is carried out in cells. Cell culture involves the growth of cells in vitro as monolayers attached to the surface of a glass or plastic culture vessel or, less frequently, as suspensions. Cell monolayers may be primary or continuous. A primary cell line is one derived from cells or tissue taken directly from the animal. Foetal tissues are frequently used as they grow well, disaggregate easily and the cells tend to be more permissive than those originating from adult tissue. Primary cell lines are labour-intensive to prepare and die or exhibit a decline in permissiveness after a limited number of passages. They have a finite life span. Thus, whenever possible, most laboratories opt to use continuous or established cell lines. These cell lines are usually available commercially and are capable of extended or indefinite propagation. They originate from malignant neoplasms or transformed cell lines. Many have a narrower range of virus susceptibilities than do primary cell lines. There is no single cell line that is susceptible to all viruses. Thus, the laboratory has to choose cell lines for routine use that are permissive for the viruses most commonly encountered. Figure 5.1 Virus isolation and identification. CAM = chorioallantoic membrane; ELISA = enzyme-linked immunosorbent assay. Virus replication in cells is detected by observing the cytopathic effect (CPE) or the formation of plaques, that is, areas of cell lysis which do not take up stain such as neutral red. Inoculated cell monolayers are observed daily by microscopy for many days. Many viruses produce a characteristic type of CPE. When no CPE is observed blind passaging of the supernatant onto a fresh monolayer may result in amplification of virus, facilitating detection. Samples that are contaminated may require filtering to remove bacteria or fungi prior to inoculation. Some viruses are non-cytopathic but can be detected by a characteristic property. For example, some viruses of the orthomyxovirus and paramyxovirus groups cause the adherence of erythrocytes to cells (haemadsorption) and some viruses agglutinate erythrocytes (haemagglutination). Non-cytopathic viruses can also be detected in cell culture supernatant by the detection of viral antigen by immunofluorescence or immunoenzyme techniques or by detection of nucleic acid by the polymerase chain reaction (PCR) (see Chapter 4). Some viruses, for example certain strains of influenza and bluetongue virus, grow more readily in embryonated eggs than in tissue culture. Fertile eggs for virus isolation should, if possible, be obtained from a specific pathogen-free flock to minimize problems with egg-transmitted viruses and maternal antibody. Before inoculation the eggs are candled to ensure the embryo is alive and to mark the position of the air sac and the major blood vessels. The eggs are inoculated between days five and 14 of incubation, depending on the route used (allantoic or amniotic cavity, yolk sac, chorioallantoic membrane or intravenous). Virus introduced into the allantoic cavity replicates in the endodermal cells of the allantois and is released into the allantoic fluid. Virus introduced into the amniotic cavity enters the respiratory and digestive tracts of the embryo and may therefore replicate in a variety of cell types depending on the tropism of the virus. These two routes are frequently used for the isolation of influenza viruses. The chorioallantoic route is used more frequently for viruses which produce plaques or pocks on the chorioallantois (Fig. 5.2), for example, fowlpox. The yolk-sac route is used for the isolation of rickettsiae and chlamydiae. Many viruses that replicate in eggs may be detected by examining the fluids or tissues harvested two to six days post inoculation. Influenza virus may be detected by the haemagglutinating activity of the harvested allantoic or amniotic fluid, and its inhibition with specific antiserum. If optimal isolation conditions for the virus in question are provided, then isolation can be a very sensitive procedure for the detection of viruses, while permitting further study of the virus. However, virus isolation is labour-intensive, expensive and slow to yield results. A number of passes may be required to adapt the virus to growth in the laboratory and special care is required in the handling and transport of specimens (see Chapter 1) to ensure the viability of viruses in the sample upon arrival at the laboratory. A number of cultivation factors influence the reliability of virus isolation (Fig. 5.3). For example, rotaviruses and coronaviruses replicate best in the presence of trypsin. Rolling the inoculated cell culture tubes may enhance virus replication and CPE. Co-cultivation techniques may be required. Certain viruses, such as alcelaphine herpesvirus-1 cannot be isolated from dead cells but may be recovered from peripheral blood leukocytes if cell viability is preserved during processing. Other viruses such as papillomaviruses have not been grown in cell culture. Figure 5.3 Factors for consideration when undertaking viral cultivation. SPF = specific pathogen-free; CAM = chorioallantoic membrane. Following isolation, additional tests are usually required to specifically identify the virus (Tables 5.1 to 5.6). Most of the techniques discussed in the chapter on serological diagnosis (Chapter 3) may be used for this purpose as may PCR (Chapter 4). Methods that require specific antisera include immunocytochemical staining, haemagglutination inhibition, complement fixation and neutralization. PCR does not require specific antisera and can be used to rapidly and specifically identify virus isolates.
The isolation of viruses and the detection of virus and viral antigens
Virus isolation
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree