CHAPTER 33 The Hematologic and Lymphoid Systems
Sample Collection
Collection of blood samples from neonatal and young animals for hematological and coagulation evaluation can be challenging because of small vessels and relatively small quantities of blood available for testing. Blood collection tubes are available from Becton, Dickinson and Company (Franklin Lakes, NJ; http://www.bd.com/vacutainer/pdfs/VS7629_ProductCat.pdf, BD Microtainer Blood Collection Tubes, BD Microtainer Plastic Clad Micro-Hematocrit Tubes, and 1.8-ml draw BD Vacutainer Citrate Tubes) that will permit collection of sufficient quantities of blood for the various hematological (0.6 ml) and coagulation (<2 ml) testing discussed in the following text.
The Hemogram
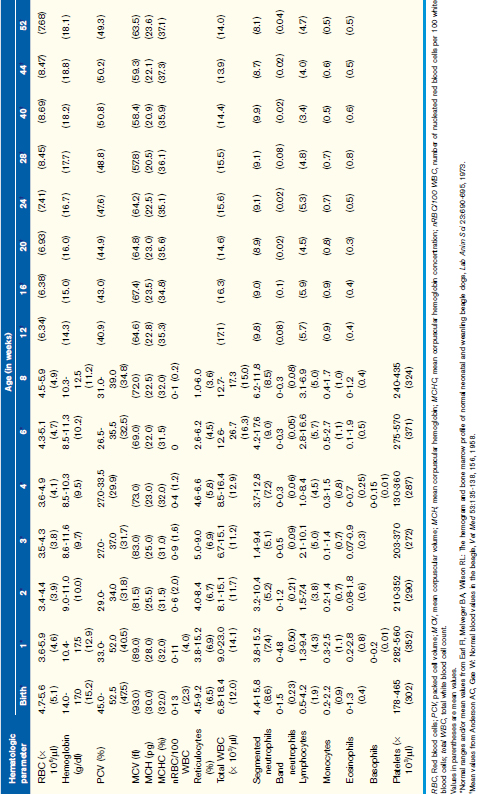
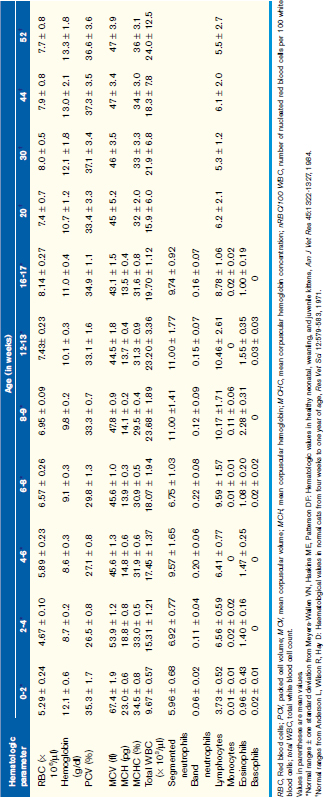
Normal Hematologic Values for the Puppy and the Kitten
Unfortunately, hemogram data have not been reported for mixed-breed puppies and kittens younger than 6 months of age, but some studies have reported data for specific breeds. A recent study reported comparison data for Beagles and Labrador Retrievers. These data were limited but did show some significant differences between these two breeds in the white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin, and hematocrit during the first year, and these differences were particularly prominent during the first 8 weeks of life. The more complete body of data has been obtained on animals from closed colonies of selected breeds, and Tables 33-1 and 33-2 are derived from the values reported in those studies. Although these data represent a more complete dataset, factors such as nutrition, environmental conditions, and health in a closed colony (i.e., research colony) may not adequately reflect the general populations of young animals. If, however, the hematologic values obtained for a puppy or kitten are outside the range of values presented here and the reference values for adult dogs and cats obtained from a reference laboratory, they can be considered abnormal with confidence.
Generally WBC counts for kittens and puppies are within the normal adult reference intervals from birth to about 6 to 8 weeks of age and vary only slightly during that period. For the dog, the WBC, segmented neutrophils, and lymphocytes are usually within the adult normal reference interval at birth, although lymphocytes may be low in some animals. These values increase between 1 and 3 months of age and may be greater than the normal adult reference interval during this time. These values will then decrease gradually over the next several months (see Table 33-1). Band neutrophils have been reported in one study to be increased at about 1 week of age and then decreased to within the adult normal reference interval thereafter. Like the dog, the WBC, segmented neutrophil, and lymphocyte counts for kittens are within the normal adult reference intervals at birth, but the WBC and lymphocyte counts increase above the normal adult reference interval between 2 and 4 months of age. These values then return to within the normal adult reference interval by about 5 to 6 months of age (see Table 33-2). It has been proposed that this increase in WBC and lymphocyte counts in kittens is associated with excitement caused during blood collection, although an increase in mature neutrophils was not documented during this period.
Erythrogram Evaluation
The complete blood count (CBC) and a blood film evaluation will provide most of the information necessary to evaluate the circulating RBC mass for changes related to disease. By far the most common and diagnostically significant hematologic change in puppies and kittens, as well as adult dogs and cats, is anemia. Anemia is not a primary disease, but determining the type of anemia along with the associated potential differential diagnoses will aid in diagnosis of the primary disease. For this discussion, anemia will be classified pathophysiologically as regenerative, iron deficiency, or nonregenerative anemia. Box 33-1 lists some possible causes of anemia in puppies and kittens.
BOX 33-1 Causes of anemia in the puppy and kitten
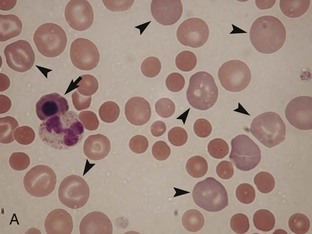
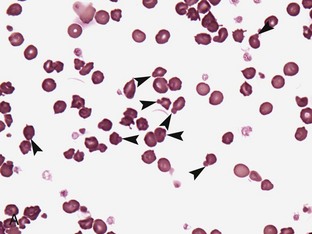
Regenerative anemia can be further classified as being caused by hemorrhage (blood loss) or hemolysis and results in a characteristic increase in RBC polychromasia and reticulocytes in circulation. Polychromasia, seen on a Romanowsky-type (Wright’s, Wright’s-Giemsa, or DifQuik) stained blood film, and reticulocytes, seen on a new methylene blue or brilliant cresyl blue-stained blood film, can be observed as early as 2 days following the onset of blood loss or hemolysis but usually require 5 to 7 days to reach a maximum response (Figure 33-1). Polychromasia is only semiquantitative and can be difficult to determine on some preparations, depending on the quality of the blood film and the stain used. A reticulocyte count, which is quantitative, is a more accurate technique for assessing the regenerative response. Nucleated RBCs (nRBCs) are not specific for a regenerative response but may be present in a regenerative response. The presence of nRBCs should not be used as the sole indicator of a regenerative response. Other indicators of an adequate bone marrow response, such as polychromasia and reticulocytes in proportion to the level of anemia, must also be present.
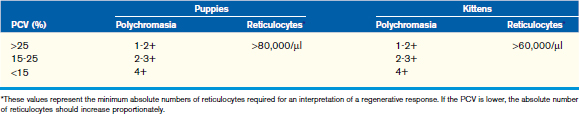
The number of reticulocytes, or polychromatophilic RBCs, necessary to determine a regenerative response depends on the PCV; the lower the PCV, the more reticulocytes (or polychromatophils) are necessary to support an interpretation of a regenerative response. The criteria used to determine adequate regeneration for adult dogs and cats can probably be used for puppies and kittens more than 4 months of age (Table 33-3), but young animals less than 4 months of age should mount a more vigorous response than the older animals. Ideally, an absolute reticulocyte concentration (Abs RC) should be used to determine regeneration (Abs RC = RBC/µl × % reticulocytes = retics/µl). For most puppies and kittens, a regenerative response can also be assessed using a corrected reticulocyte percentage (cRP = RP × patient Hct/avg Hct). Normal canine values for corrected reticulocyte counts during the first year of life can be found in Table 33-1.
Once it has been determined that the patient has a regenerative anemia, the total plasma (or serum) protein can be used to aid in differentiating between blood loss and hemolysis. Because plasma will be lost from the body with hemorrhage, the total plasma protein usually will be low. In contrast, for hemolytic anemia, the plasma remains in the body, and total plasma protein will be normal to increased. The magnitude of the decrease in total plasma protein associated with hemorrhage will be associated with the severity and duration of the hemorrhage. Some causes of hemorrhagic regenerative anemia are shown in Box 33-1. With continued hemorrhage, iron stores are lost, leading to decreased hemoglobin synthesis in developing erythrocytes. Because hemoglobin concentration determines the end of the division phase of erythrocyte development, decreased iron stores will lead to increased cell division resulting in microcytic RBCs with decreased concentrations of hemoglobin (hypochromasia). Hypochromasia is a hallmark of iron deficiency. Even if RBCs do not look smaller, the hypochromasia will be evident as cells with larger pale centers with a thin ring of hemoglobin at the periphery of the cells.
In both kittens and puppies, hemolytic anemia can have a number of causes (see Box 33-1). To date, there is no evidence that kittens and puppies are more prone to hemolytic anemia than adult animals. Although not common, neonatal isoerythrolysis is the most common type of immune-mediated hemolytic anemia in newborn kittens and is related to their blood type (types A, B, AB). Neonatal isoerythrolysis is covered in greater detail in Chapter 2. Briefly, hemolysis does not manifest itself during gestation or at birth, but results when a blood type A kitten receives colostrum containing anti-type A alloantibodies from the blood type B queen. Affected kittens may exhibit lethargy progressing to depression, lost suckle reflex, anemia, icterus, and hemoglobinuria followed by death in 2 to 3 days if not treated. Not all type A kittens born to type B queens will necessarily be affected, however. Specific blood types are associated with specific breeds and specific areas of the world. Neonatal isoerythrolysis is most common in Cornish and Devon Rex, Exotic, British Shorthair, and Persian cats, but has also been reported in Himalayan and in domestic shorthair and longhair cats. Neonatal isoerythrolysis is rare in puppies.
RBC morphology observed on a stained blood film is valuable in determining possible causes of hemolytic anemia. A summary of some of the most common RBC changes in association with the causes of hemolytic anemia are given in Table 33-4. In dogs, immune-mediated hemolytic anemia (IMHA) is suspected when spherocytes are seen on the blood film of dogs. Because cat RBCs do not show a consistent central pallor and are smaller than dog RBCs, spherocytes are more difficult to identify on blood films from cats. In addition to the intravascular and extravascular hemolysis that occurs in cases of IMHA, RBC agglutination and/or a positive direct Coombs test are often detected.
TABLE 33-4 Morphologic features of RBCs useful for diagnosis of hemolytic anemias
| Cause of hemolysis | RBC feature | Description of RBC abnormality |
|---|---|---|
| Immune-mediated | Agglutination | Small to large clumps of RBCs that are not dispersed with an equal volume of normal saline |
| Spherocytes | Small RBCs with no central pallor (may be difficult to distinguish from normal RBCs in the cat) | |
| Hemoglobin oxidation | Heinz bodies | One to several spherical structures within or protruding from the RBC membrane; nonstaining and refractile with DifQuik, nonstaining to pale staining to normal hemoglobin staining with Wright’s stain, blue with new methylene blue stain |
| Microangiopathies | Schistocytes | Irregular fragments of RBCs |
| Helmet cells (keratocytes) | Football helmet-shaped RBCs with strap-like extensions of RBC membrane from each side | |
| Blister cells | RBCs with eccentric vacuoles, often at the periphery of the cell | |
| Hemoparasites | Mycoplasma spp. | Dark staining, very small, round to ring-shaped to rod-shaped organisms, usually dotted epicellularly along the periphery or across the surface of the RBC |
| Babesia | Large pear-shaped (piriform) structures, present singly, in pairs, or tetrads in the RBC | |
| Cytauxzoon | Tiny, densely stained to piriplasm-type organisms within the RBC; usually single, but tetrads have been reported |
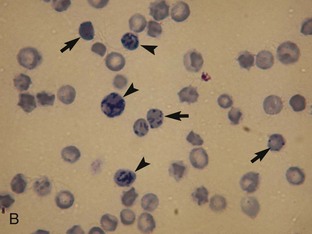
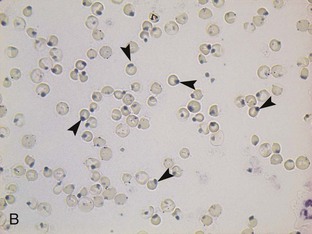
Oxidation of hemoglobin caused by some foods (e.g., onions), food additives (e.g., propylene glycol), drugs, and plant derivatives leads to hemoglobin denaturation and Heinz body formation. Heinz bodies (Figure 33-2) appear as small, sometimes clear, spherical protrusions (Romanowsky type-stained blood films) or blue spheres (new methylene blue-stained blood films) at the surface of RBCs. Heinz bodies also have been strongly correlated to some diseases, such as diabetes mellitus, hyperthyroidism, and lymphoma in cats, but these diseases are uncommon in kittens. Depending on the extent of hemoglobin denaturation, and thus the numbers of Heinz bodies, the resulting hemolytic anemia may be mild to severe. Heinz bodies are removed by the spleen, which may result in spherocytosis, especially apparent in dogs.
Leukogram Evaluation
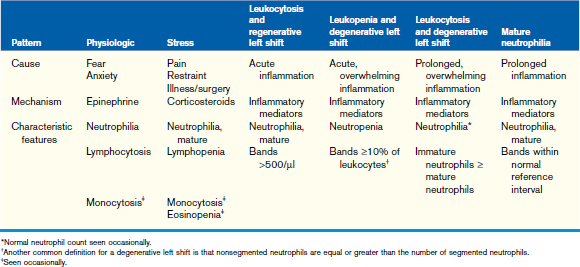
Changes in the leukogram generally consist of patterns indicative of a disease process or physiologic response in a puppy or kitten. Even though a disease process may exist, the leukogram may not necessarily reflect expected changes, and a single blood sample may not always reflect the changes occurring in the tissues. Although the presence of a specific leukogram pattern is helpful clinically, the absence of changes in the leukogram does not necessarily rule out a physiologic or pathologic process. Changes in the leukogram in response to inflammation, fear/anxiety, and stress (Table 33-5) are similar to those seen in adult animals, so these changes will be discussed only briefly here.
The inflammatory leukogram provides clinically significant information about inflammatory processes within the body, and the type of response may provide additional information about the quality and severity of the inflammatory process (see Table 33-5). In normal puppies and kittens with adequate bone marrow reserves, the most common response to inflammation is a regenerative response, characterized by a mature neutrophilia and a mild increase in band neutrophils (regenerative left shift). As a general rule, the bone marrow is functioning normally if a mature neutrophilia or a regenerative left shift is detected. In the presence of an overwhelming inflammatory process (e.g., a highly virulent infectious agent), the bone marrow may be unable to respond adequately, either because the bone marrow has been compromised or because of overwhelming destruction of cells in the tissues; this response is categorized as a leukopenic degenerative left shift. If the bone marrow has sufficient time to respond, increased numbers of mature neutrophils may be seen on the blood film, but cells representing immature stages of neutrophil maturation (typically bands, metamyelocytes, and myelocytes) are still in numbers equal to or greater than those of the mature cells seen; this is termed a leukocytic degenerative left shift. Both leukopenic and leukocytic degenerative left shifts alert the clinician to the severity of the patient’s condition and the need to take urgent action—identification of the inciting cause and initiation of treatment as soon as possible are critical.
Many texts present information regarding normal and abnormal features of the various leukocytes (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) seen on a blood film. The changes seen in the cells in response to numerous diseases do not differ from the responses seen in adult animals and so will not be covered in detail here. To aid the clinician in generating differential diagnoses, Boxes 33-2 to 33-5 are provided as quick references for causes of neutrophilia/neutropenia, monocytosis, eosinophilia, and basophilia, respectively. A similar table will be provided for lymphocytes in a later section of this chapter.
BOX 33-2 Causes of neutrophilia and neutropenia
†Usually associated with primary diseases, such as renal failure, endocrinopathies, inflammation, infection (especially viruses), and neoplastic disease.
BOX 33-4 Causes of eosinophilia*
Parasites
Idiopathic Eosinophilic Syndromes
Hypoadrenocorticism (Caused by Maldevelopment of the Adrenal Cortex)
Note: The list serves only to provide examples of possible causes.
Heritable Diseases of Puppies and Kittens Resulting in Hemogram Changes
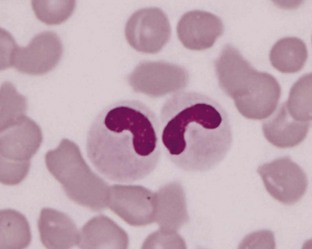
Pelger-Huët anomaly is an autosomal dominant disorder characterized by hyposegmentation of the nuclei of neutrophils, basophils, and eosinophils (Figure 33-3). Hyposegmented neutrophils often resemble bands or even metamyelocytes and myelocytes but have coarse mature chromatin and normal neutrophil function. Affected animals are usually healthy, and the anomaly is discovered incidentally when evaluating blood films; however, homozygous animals may be stillborn. Pelger-Huët anomaly has been reported in domestic shorthaired cats and various dog breeds (Australian Shepherd, Australian Blue Heeler, Basenji, Boston Terrier, Coonhounds, Cocker Spaniel, English-American Foxhound, German Shepherd Dog, Samoyed, and cross-breed dogs).
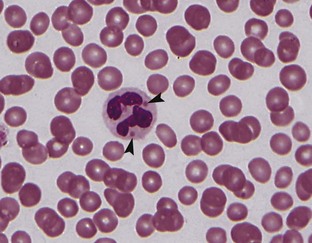
Chédiak-Higashi syndrome, an inherited autosomal recessive disorder, is found primarily in “blue smoke” Persian cats. The syndrome is characterized by abnormally large primary granules in granulocytes (Figure 33-4), large lysosomes in lymphocytes and other cell types (e.g., liver and kidney), large melanin granules in melanocytes, an increased susceptibility to infection, especially in the upper respiratory tract, and septicemia. In addition to coat color changes, affected cats have light-colored irises and red fundic light reflections, which cause them to be photophobic. Cataracts also form at an early age. Although coagulation screening tests are normal and platelet numbers are normal, platelet function may be abnormal, resulting in hematomas at venipuncture sites and bleeding following even minor surgery. Treatment involves supportive and symptomatic care, but allogenic bone marrow transplants have successfully corrected the neutrophil migration defect and platelet storage pool deficiency associated with Chédiak-Higashi syndrome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree