Chapter 19 The Genera Pseudomonas and Burkholderia
The genus Pseudomonas was described more than a century ago and has been completely revised on multiple occasions because of taxonomic heterogeneity. The nomenclatural arrangements eventually led to creation of the genus Burkholderia. Recently proposed taxonomic changes include placement of Burkholderia into the class β-Proteobacteria, order Burkholderiales, family Burkholderiaceae. Pseudomonas remains in the class γ-Proteobacteria, order Pseudomonadales, family Pseudomonadaceae. Both genera include aerobic, non–spore-forming, oxidase-positive, nonfermentative, gram-negative rods that grow on MacConkey agar. Members of these genera are versatile pathogens (Table 19-1).
TABLE 19-1 Diseases and Primary Hosts of the Veterinary Significant Pseudomonadaceae and Burkholderiaceae
| Organism | Host(s) | Disease |
|---|---|---|
| Pseudomonas aeruginosa | Cattle | Mastitis; abortion |
| Dog | Otitis externa | |
| Horse | Corneal ulcer; metritis | |
| Mink | Hemorrhagic pneumonia | |
| Poultry | Embryo mortality | |
| Sheep | Fleece rot | |
| Captive snakes | Necrotic stomatitis | |
| Many animals | Urinary tract infections; septicemia; wound infections; abscesses; granulomas (botryomycosis) | |
| Pseudomonas fluorescens | Cattle | Mastitis |
| Fish | Tail/fin rot, septicemia | |
| Poultry | Embryo mortality | |
| Burkholderia mallei | Mule, donkey | Acute glanders |
| Horse | Chronic glanders | |
| Cat, dog | Acute glanders | |
| Burkholderia pseudomallei | Cat, cattle, dog, horse, marine mammals, pig, ruminants | Melioidosis (chronic nodular form more common than acute septicemia) |
THE GENUS PSEUDOMONAS
Pseudomonas spp. have worldwide distribution. They are ubiquitous in soil, water, decaying organic matter, and vegetation, but are opportunistic pathogens of animals, plants, and humans. One species, Pseudomonas aeruginosa, is commonly encountered as an animal pathogen and is particularly noteworthy for its ability to cause disease in susceptible hosts and its resistance to antibiotics. Another species, Pseudomonas fluorescens, is occasionally isolated from veterinary specimens.
DISEASES AND EPIDEMIOLOGY
Pseudomonas aeruginosa is often recovered from dead poultry embryos and newly hatched chicks. Severe disease outbreaks have followed egg injection with contaminated vaccines or egg-dipping in contaminated antimicrobial solutions. Contamination typically results from poor handling of these products rather than from the products themselves.
Botryomycosis is a granulomatous disease of the skin, subcutis, and viscera. The term was used initially because of the histologic resemblance of the lesions to fungal granulomas but is erroneous because the etiology is bacterial rather than mycotic. Most recorded cases of botryomycosis are attributed to Staphylococcus aureus; however, P. aeruginosa has also occasionally been implicated. Pseudomonal botryomycosis has been described in cattle, laboratory rodents, and man. Trauma is an important prerequisite for inoculation of the bacterium into tissues. In cattle, lesions have been reported on the udder and in the nasopharynx. Pulmonary disease has occurred in guinea pigs. Microscopic examination of lesions reveals pyogranulomas surrounding colonies of gram-negative rods. Granules of eosinophilic material with peripheral, radiating clubs (Splendore-Hoeppli phenomenon) form around the bacterial colonies and are surrounded by neutrophils, eosinophils, and macrophages (Figure 19-1). The granules resemble those of actinomycosis.
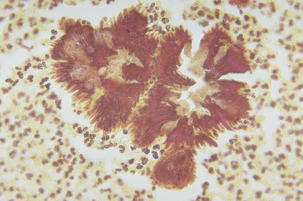
FIGURE 19-1 Botryomycosis lesions in tissue associated with Pseudomonas aeruginosa.
(Courtesy Public Health Image Library, PHIL #4260, Centers for Disease Control and Prevention, Atlanta, 1973, Lucille K. George.)
PATHOGENESIS
Pseudomonas aeruginosa possesses a wide variety of virulence factors, including pili, capsule, endo toxin, pyocyanin, hemolysins, enzymes, toxins, and an inherent resistance to many antimicrobials. These cause extensive tissue damage at or near the site of infection, cause permanent disruption of host cell membranes, and interfere with immune defense mechanisms. It is difficult to define the specific role for each factor in disease because most investigators believe multiple factors contribute to virulence (Table 19-2).
TABLE 19-2 Virulence Factors of Pseudomonas aeruginosa
| Virulence Factors | Biologic Effect(s) |
|---|---|
| Alkaline protease | Inactivates interferon and tumor necrosis factor; causes tissue damage |
| Antibiotic resistance | Complicates chemotherapy |
| Capsule | Protects organism from phagocytosis and antibiotic penetration; functions as an adhesin |
| Cytotoxin | Inhibits leukocyte function; disrupts pulmonary microcirculation |
| Elastase | Damages blood vessels, skin, and pulmonary tissue; degrades complement components; inhibits neutrophil chemotaxis |
| Endotoxin | Mediates biologic effects of sepsis and inflammation |
| Exotoxin A | Inhibits cellular protein synthesis; causes tissue necrosis; has immunosuppressive effects |
| Exotoxins S and T | Inhibit cellular protein synthesis; have immunosuppressive effects; facilitate tissue invasion |
| Phospholipase C | Hemolysin that stimulates inflammation; causes tissue damage |
| Pili | Mediate adhesion to host cells |
| Pyocyanin | Pigment that interferes with the mucociliary apparatus; produces toxic oxygen radicals that mediate tissue damage |
| Rhamnolipid | Hemolysin with lecithinase activity that damages host cell membranes; inhibits the mucociliary apparatus; inhibits macrophage function |
Because of the opportunistic nature of pseudomonal infections, the first step in pathogenesis in any site is a breach in the host defenses, such as skin trauma, disruption of normal microflora from antimicrobial therapy, or other circumstances. The next step is bacterial adherence to host epithelial cells via pili and capsular polysaccharide. Capsule further protects the organism from phagocytosis by host immune cells. Proteases promote dissemination of P. aeruginosa in the tissues. Elastase destroys elastin, disrupting the integrity of host cellular basement membranes and removing physical barriers that would normally inhibit the spread of infection. Elastin is also a major component of lung and vascular tissue. Elastase likely plays a large role in the pathogenesis of mink hemorrhagic pneumonia by damaging lung parenchyma and blood vessels. Toxins and proteases are responsible for edema, hemorrhage, and necrosis that occur in skin wounds. In particular, alkaline protease facilitates the dissemination of bacteria and causes tissue damage; phospholipase C degrades cellular membranes by way of its lecithinase activity. The pathogenesis of ulcerative keratitis is mediated by endotoxin, exotoxins, and proteases. Endotoxin attracts and activates polymorphonuclear cells, and corneal inflammation and subsequent tissue damage results from release of oxidative substances by the neutrophils. Pseudomonal exotoxins are also directly responsible for destruction of corneal epithelial cells. Proteases are thought to be crucial for the development of ulcerative keratitis, but their precise role remains to be determined. Pseudomonads have enzymes that are capable of digesting eggshell cuticle in conditions of high humidity, resulting in invasion of the embryo.



